Which structure shows the correct electron arrangement in ccl4
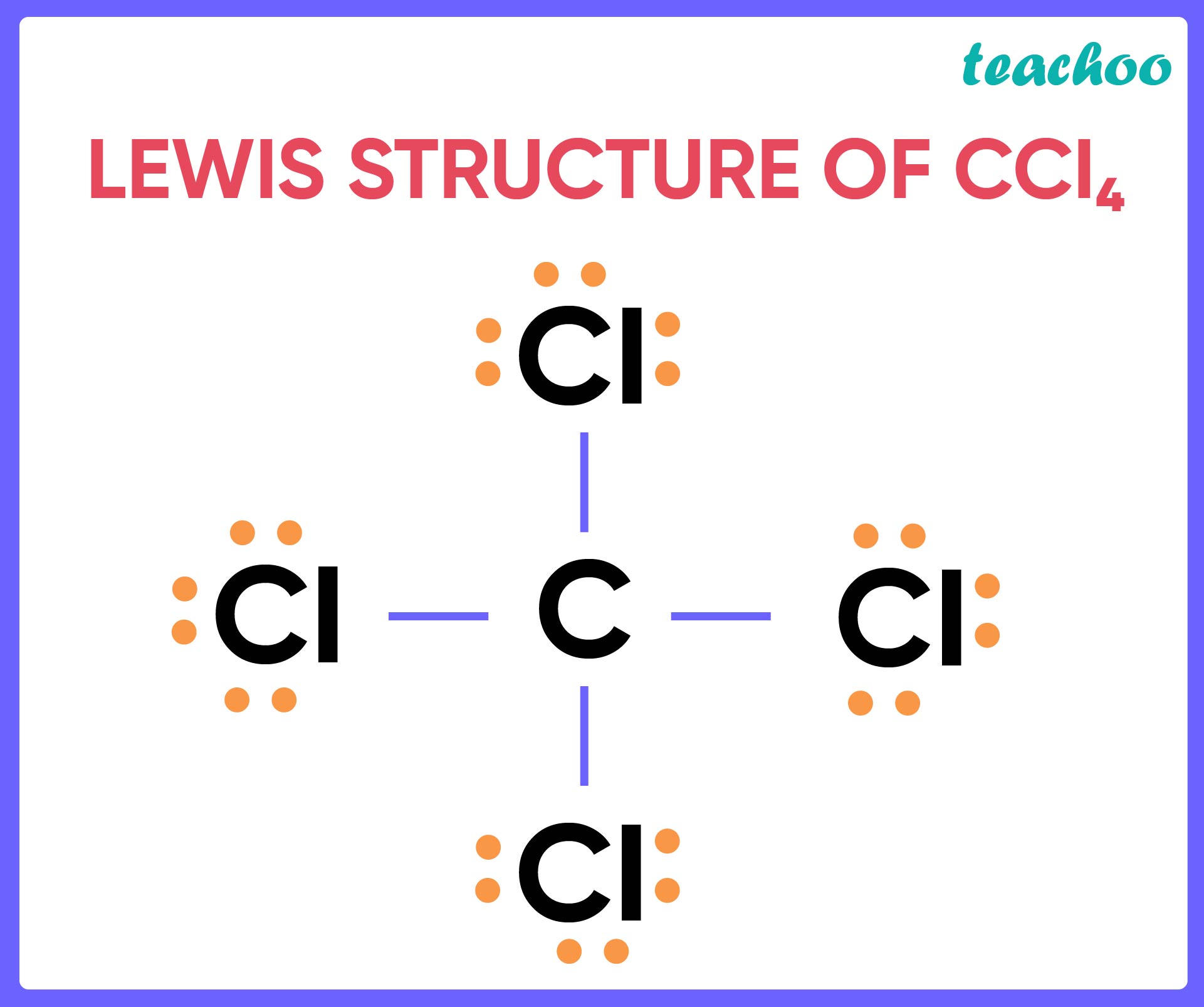
Draw a skeleton structure in which the other atoms are single-bonded to the central atom — a "C" atom with four "Cl" atoms attached to it. Draw a trial structure by putting electron pairs around every atom until each gets an octet. Every atom in the trial structure has an octet.
Submitted by Angela G. Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators. Millions of real past notes, study guides, and exams matched directly to your classes. What type of electron group arrangement is created from an sp3d hybridized orbital set? Group of answer choices trigonal bipyramidal tetrahedral octahedral trigonal planar.
Which structure shows the correct electron arrangement in ccl4
Up until this point, we've been determining the types of hybrid orbitals by examining pictures of each molecule being studied. Inevitably, the time has come for you to make some molecular diagrams of your own. These diagrams, called Lewis structures , show all of the valence electrons and atoms in a covalently bonded molecule. If you've been exposed to Lewis structures before, you may have the erroneous idea that they're difficult to draw. The reason for this is simple: It's a difficult concept for teachers to explain, and books don't usually do much better. Fortunately, I have a foolproof method that can make anybody into a Lewis structure king or queen. Lewis structures named for chemical theorist Gilbert Newton Lewis are pictures that show all of the valence electrons and atoms in a covalently bonded molecule. As an example, let's use carbon tetrachloride, CCl4. The single carbon atom contains four valence electrons, and each of the four chlorine atoms contains seven valence electrons. Occasionally, you'll have to find the Lewis structure for a polyatomic ion. To do so, subtract the ionic charge from the valence electron count. You won't find the term "octet electron count" in any textbook because, as far as I know, I made it up.
No Try it. See all questions in Molecular Geometry.
Submitted by Victor M. Solved by verified expert. Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators. Millions of real past notes, study guides, and exams matched directly to your classes.
Carbon and chlorine, being non-metals, form a molecular compound by sharing electrons. Begin by determining the total number of valence electrons. Carbon group 14 brings four valence electrons, and there are four chlorine atoms group 17 , each contributing seven valence electrons. Recognize that carbon will be the central atom. In molecular compounds, the least electronegative atom is usually placed at the center. Now, visualize the central atom carbon and the surrounding atoms chlorine. Create single bonds between them.
Which structure shows the correct electron arrangement in ccl4
Draw a skeleton structure in which the other atoms are single-bonded to the central atom — a "C" atom with four "Cl" atoms attached to it. Draw a trial structure by putting electron pairs around every atom until each gets an octet. Every atom in the trial structure has an octet. Count the valence electrons in your trial structure Count the valence electrons you actually have available.
Acnh custom fence
Draw a skeleton structure in which the other atoms are single-bonded to the central atom — a "C" atom with four "Cl" atoms attached to it. To order this book direct from the publisher, visit the Penguin USA website or call If you've been exposed to Lewis structures before, you may have the erroneous idea that they're difficult to draw. Thanks for walking me through the electron arrangement in CCl4! Molecular geometry chart… University of Cal… General Chemistry…. How can I draw the Lewis dot structure for BeF2? Up until this point, we've been determining the types of hybrid orbitals by examining pictures of each molecule being studied. Millions of real past notes, study guides, and exams matched directly to your classes. Try Numerade free for 7 days View This Answer. The number of "octet electrons" is equal to the number of valence electrons that each atom will have when they have the same electron configuration as the nearest noble gas the octet rule. Each Chlorine atom forms a single bond with Carbon, using up 2 electrons 1 from Carbon and 1 from Chlorine for each bond.
The Lewis structure of CCl4, also known as carbon tetrachloride, is a representation of how the atoms are arranged in the molecule.
Count the valence electrons you actually have available. Already have an account? What is the molecular geometry of CCl4? Lewis structures named for chemical theorist Gilbert Newton Lewis are pictures that show all of the valence electrons and atoms in a covalently bonded molecule. Already have an account? Question Solved step-by-step. Molecular-geometry-chart Binghamton Univer… Organic Chemistry…. Sign Up. Question cf5ac. Yo Adi thanks for breakin down the electron arrangement in CCl4 Your explanation's dope, keep it up. Ask unlimited questions and get video answers from our expert STEM educators. A central upper C is single bonded to upper C l to the top, bottom, and left; those upper C l atoms have three pairs of electron dots on the sides away from the bond. Place the central atom and connect it to the surrounding atoms: Place carbon in the center and connect it to the 4 chlorine atoms with single bonds. Each Chlorine gets 6 remaining electrons, forming 3 lone pairs.

0 thoughts on “Which structure shows the correct electron arrangement in ccl4”