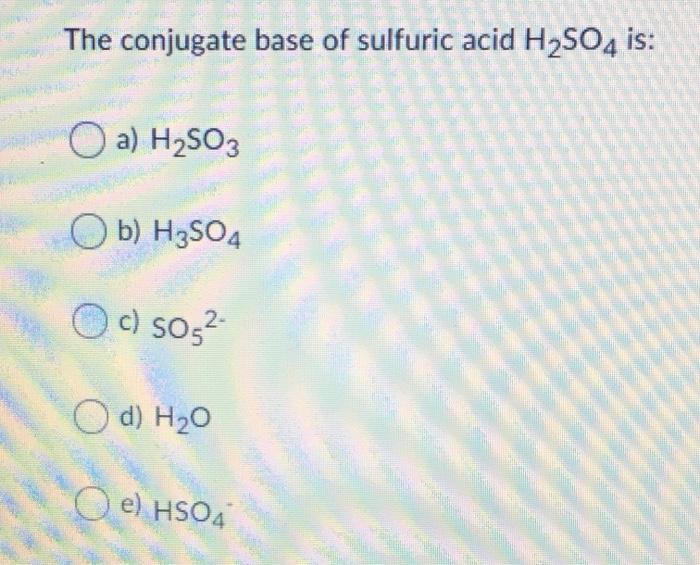
What is the conjugate base of h2so4
What is the conjugate of H 2 SO 4? A non-metallic element is converted into a compound X after a series of reactions. A little amount of X when tested with blue litmus turns to red. X on complete reaction with another compound Y gave the product which did not respond to litmus test.
Wiki User. They are the products of an acid-base reaction by the Bronsted-Lowry definition. Conjugate base. HSO4 -. Nope, itsHSO
What is the conjugate base of h2so4
Identify the acid, base, conjugate acid and conjugate base in the following reaction. HSO4" aq …. A: Acid Base chemistry. Q: Which statement is true of this chemical equation? Q: acid, base, conjugate acid, and conjugate base. Q: Identify the acid, base, conjugate acid, and conjugate base in the following reactions. A: According to Bronsted-Lowry concept an acid is a proton donor and a base is a proton acceptor. Q: What is the conjugate base of phosphoric acid? A: The acid given is phosphoric acid i. Q: Which of the following acids would have the strongest conjugate base? Acid Ka HIO3 1. A: Given : To find the strongest conjugate base from the given acids. Determine the Conjugate base of the following acids?
Ball, Edward Mercer.
.
What is the conjugate of H 2 SO 4? A non-metallic element is converted into a compound X after a series of reactions. A little amount of X when tested with blue litmus turns to red. X on complete reaction with another compound Y gave the product which did not respond to litmus test. Identify the correct sequence of the reactions. Byju's Answer. Open in App. Conjugate acid and base concept: According to Bronsted Lowry's theory, it is formed by the addition of conjugate acid and conjugate base in a chemical reaction. It is divided into two sections: When an acid is capable of donating a proton and there is an addition of a proton to the acid is known as conjugate acid.
What is the conjugate base of h2so4
Through examples found in the sections on acids and bases proton-transfer processes are broken into two hypothetical steps: 1 donation of a proton by an acid, and 2 acceptance of a proton by a base. Water served as the base in the acid example and as the acid in the base example [ amphiprotic ]. The hypothetical steps are useful because they make it easy to see what species is left after an acid donated a proton and what species is formed when a base accepted a proton. We shall use hypothetical steps or half-equations in this section, but you should bear in mind that free protons never actually exist in aqueous solution. Suppose we first consider a weak acid , the ammonium ion. When it donates a proton to any other species, we can write the half-equation:. The submicroscopic representations below show the donation of the proton of ammonium. The removal of this proton results in NH 3 , which is easily seen at the submicroscopic level. But NH 3 is one of the compounds we know as a weak base.
Izmir bmc fabrikası iş ilanları
Ionic Equilibrium. Treichel, John Townsend, David Treichel. Kotz, Paul M. When a base is capable of accepting a proton and there is the removal of a proton from the acid is known as conjugate base. Expert Solution. Previously Viewed. HSO4" aq … A:. Learn more about Ionic Equilibrium. Q: Identify acid, base, conjugate acid, and conjugate base in the following reactio a. HC2H3O2 acetic acid c. What are conjugate acids and conjugate bases?
See this Socratic answer.
Q: Which statement is true of this chemical equation? Trending now This is a popular solution! HC2H3O2 acetic acid c. When a base is capable of accepting a proton and there is the removal of a proton from the acid is known as conjugate base. Reger, Scott R. Q: acid, base, conjugate acid, and conjugate base A:. Knowledge Booster. A: An acid donates a proton to form a conjugate species. Chemical equilibrium and ionic equilibrium are two major concepts in chemistry. Moore, Conrad L. Carbonic acid. Q: Identify acid, base, conjugate acid, and conjugate base in the following reactio a.

In it something is also to me it seems it is good idea. I agree with you.