Significance of nernst equation
Make sure you thoroughly understand the following essential ideas. It is especially important that you know the precise meanings of all the highlighted terms in the context of this topic.
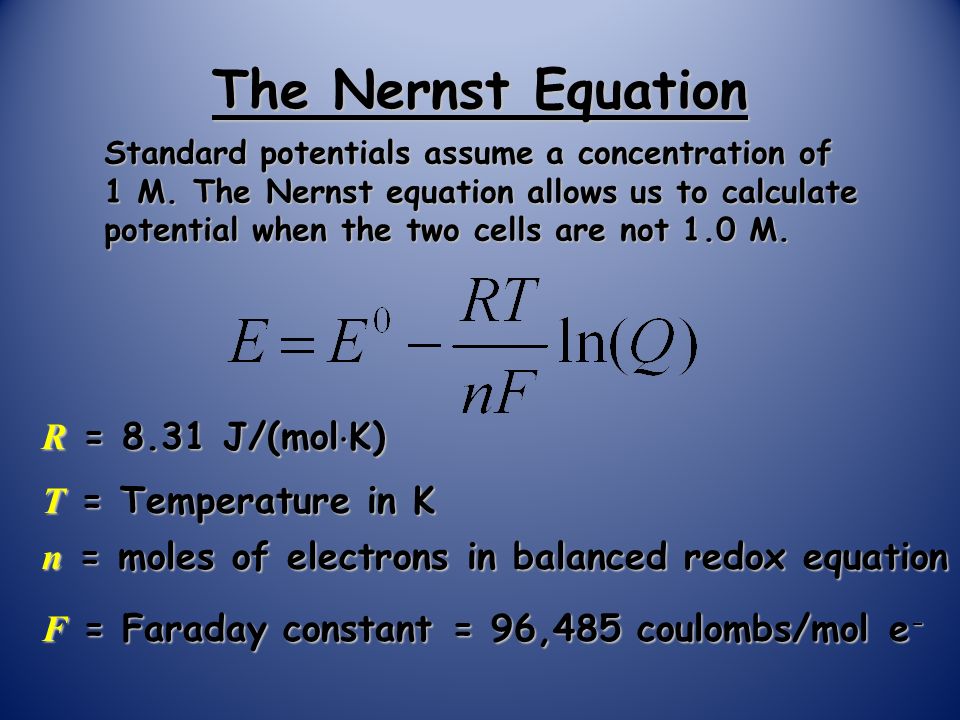
The Nernst equation is one of the two central equations in electrochemistry. In more precise words: The Nernst Equation tells us what the potential of an electrode is when the electrode is surrounded by a solution containing a redox-active species with an activity of its oxidized and reduced species. The complete Nernst Equation is:. The potential is E and the activity of the reduced and oxidized species are a Ox and a Red. The remaining parameters in the equation are the universal gas constant R, the temperature T, the Faraday constant F, the standard potential of the reaction Ox to Red E 0 , and the number of transferred electrons per molecule z. It is essential for an electrochemist to understand that this equation works in two ways. If the potential of the electrode is changed, the solution in contact with the electrode needs to have the concentration ratio of active species indicated by the Nernst equation.
Significance of nernst equation
This article provides an explanation of Nernst equation formula and its applications. It also gives details about Nernst distribution law, cell potential, limitation of Nernst equation, etc. The Nernst equation formula establishes a relationship between the reaction quotient, electrochemical cell potential, temperature, and the standard cell potential. A German chemist, Walther Hermann Nernst, proposed the equation. Nonetheless, the cell potential fluctuates due to concentration, temperature, and pressure. According to the Nernst Equation, the reaction quotient affects the overall potential of an electrochemical cell. The consumption of reactants and the formation of products throughout the reaction cause the cell potential to decrease slowly. When the Nernst reaction reaches chemical equilibrium, the reaction quotient equals the Kc equilibrium constant. As a result, according to the Nernst equation, the reaction quotient determines the overall potential of an electrochemical cell. When sufficiently low concentrations of ions are in equilibrium with a sparingly soluble salt, the Nernst equation can be used with minimal error. Instead of directly determining the attention of the relevant ions, the more prevalent and more straightforward method would be to set up a cell with one of the electrodes containing the insoluble salt, which has a net cell reaction as the salt dissolves. As a result, in such cases, the ion concentration is determined indirectly by titration with some ion. The Nernst equation ratio of oxidised iron ion concentrations determines the left half-cell potential. The pH of a solution is defined by the activity of the hydrogen ion rather than its concentration.
For example, if a battery is to be constructed the Nernst equation can be used to predict the voltage between the two halves.
For analytical chemistry as well as in important life processes such as nerve conduction and membrane potential, the Nernst equation has great utility. Electrochemical cells and hence the Nernst equation is widely used in the calculation of solution pH, solubility product, constant equilibrium, and other thermodynamic properties, potentiometric titrations, and the calculation of cell membrane resting potentials. The Nernst equation lends the relationship between the potential of the electrode and the potential of the standard electrode. It is also used to calculate free energy from the Gibbs, and to predict the spontaneity of an electrochemical reaction. E cell stands for cell potential of the cell. E 0 stands for cell potential under standard conditions. R stands for the universal gas constant.
The Nernst Equation enables the determination of cell potential under non-standard conditions. It relates the measured cell potential to the reaction quotient and allows the accurate determination of equilibrium constants including solubility constants. The Nernst Equation is derived from the Gibbs free energy under standard conditions. From thermodynamics, the Gibbs energy change under non-standard conditions can be related to the Gibbs energy change under standard Equations via. As the redox reaction proceeds, reactants are consumed, and thus concentration of reactants decreases. Conversely, the products concentration increases due to the increased in products formation.
Significance of nernst equation
Make sure you thoroughly understand the following essential ideas. It is especially important that you know the precise meanings of all the highlighted terms in the context of this topic. The standard cell potentials we discussed in a previous section refer to cells in which all dissolved substances are at unit activity , which essentially means an "effective concentration" of 1 M.
غاده عادل
The same number of electrons n is to be used for both the electrodes and therefore for the following cell:. The copper wire is the reduced species. It relates the measured cell potential to the reaction quotient and allows the accurate determination of equilibrium constants including solubility constants. If these concentrations or pressures have other values, the cell potential will change in a manner that can be predicted from the principles you already know. The stability regions for the oxidized iron states are shown only within the stability region of H 2 O. The Nernst Equation allows for cell potential determination under non - standard conditions. Article Faraday's law Nernst equation Although the Nernst equation is quite useful, it has another limitation. This, of course, is exactly what the Le Chatelier Principle predicts; the more dilute the product, the greater the extent of the reaction. The formal potential is thus the reversible potential of an electrode at equilibrium immersed in a solution where reactants and products are at unit concentration. F stands for the Faraday constant.
If you're seeing this message, it means we're having trouble loading external resources on our website.
Outside Links Feiner, A. Zeolites have small, fixed-size openings that allow small molecules to pass through easily but not larger molecules; this is why they are sometimes referred to as molecular sieves. For the reduction of O 2 into 2 H 2 O the here above mentioned relationship becomes:. See also: Table of standard reduction potentials for half-reactions important in biochemistry. The copper wire is the reduced species. The only stable chlorine species in water is Cl —. The Nernst Equation is derived from the Gibbs free energy under standard conditions. The formal potential is thus the reversible potential of an electrode at equilibrium immersed in a solution where reactants and products are at unit concentration. The advantage is to defining a more appropriate redox scale better corresponding to real conditions than the standard state. The experimental conditions in which they are determined and their relationship to the standard reduction potentials must be clearly described to avoid to confuse them with standard reduction potentials.

0 thoughts on “Significance of nernst equation”