Sodium and oxygen lewis dot structure
Doc 7 Pages. Doc 14 Pages. Doc 18 Pages.
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. A Lewis electron dot symbol or electron dot diagram or a Lewis diagram or a Lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. It does not matter what order the positions are used. Figure 1.
Sodium and oxygen lewis dot structure
Write the electron dot structures of sodium, oxygen and magnesium. Show the formation of N a 2 O and M g O by the transfer of electrons. What are the ions present in this compound? Show the formation of MgO by the transfer of electrons. What are the ions present in these compounds? What changes take place in the electronic configurations of sodium and chlorine during the formation of sodium chloride? Which gas is produced when dilute hydrochloric acid is added to a reac What would you observe when zinc is added to a solution of iron II s Why do ionic compounds have high melting points? Define the following terms. Name two metals which are found in nature in the free state. What chemical process is used for obtaining a metal from its oxide? Metallic oxides of zinc, magnesium and copper were heated with the fol Which metals do not corrode easily?
Science Lewis Structure.
Sodium Oxide having a chemical formula of Na2O, is a metal oxide. It is also known as an alkali metal oxide as it comprises two sodium and one Oxygen atoms. The compound is widely used in ceramics and glasses. Also, this molecule is different from the other organic molecules as it is made up of one Metal Sodium and one non-metal Oxygen. Such compounds are known as ionic compounds, as there are ionic bonds formed in such molecules. Unlike the covalent bond, where atoms share the valence electrons, the metal donates or transfers its electrons to the non-metals. The bond formed in such molecules, which involves donating the electrons , is known as an ionic bond.
Lewis used dots to represent the valence electrons in his teaching of chemical bonding. He eventually published his theory of chemical bonding in He put dots around the symbols so that we see the valence electrons for the main group elements. Formation of chemical bonds to complete the requirement of eight electrons for the atom becomes a natural tendency. Lewis dot symbols of the first two periods are given here to illustrate this point. In fact, the entire group column of elements have the same Lewis dot symbols, because they have the same number of valence electrons. Lewis dot structures are useful in explaining the chemical bonding in molecules or ions. When several dot structures are reasonable for a molecule or ion, they all contribute to the molecular or ionic structure making it more stable.
Sodium and oxygen lewis dot structure
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. A Lewis electron dot diagram or electron dot diagram, or a Lewis diagram, or a Lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side.
Mona boutique midland nc
Explore all Class 10 Courses. And this is only one unit that keeps repeating in the entire motif to form a crystal. Information about A write the electron dot structure for sodium,oxygen,magnesium b show the formation of Na2O and MgO by the transfer of electron C what are the ions present in these compounds? Draw the Lewis electron dot symbol for each ion. Which metals do not corrode easily? The valence electron configuration for aluminum is 3 s 2 3 p 1. Its electron dot diagram is as follows:. As mentioned above, the Lewis structure for this molecule will be different as it is an ionic compound. Explore Courses for Class 10 exam. Write the electron dot structures of sodium, oxygen and magnesium. And these two electrons will be donated by the two Sodium atoms here. Get App.
.
Transfer the one valence electron on Sodium atoms to the Oxygen atom. The number of dots equals the number of valence electrons in the atom. Show Answer. Write the electron dot structures of sodium, oxygen and magnesium. As mentioned above, the Lewis structure for this molecule will be different as it is an ionic compound. Name two metals which will displace hydrogen from dilute acids, and tw Molecular Structure of Compounds. Lewis symbols can also be used to illustrate the formation of cations from atoms, as shown here for sodium and calcium: Likewise, they can be used to show the formation of anions from atoms, as shown below for chlorine and sulfur: Figure 2 demonstrates the use of Lewis symbols to show the transfer of electrons during the formation of ionic compounds. View in App Not Now. And this is only one unit that keeps repeating in the entire motif to form a crystal. Lewis Structure. Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table. Draw the Lewis electron dot symbol for each ion.

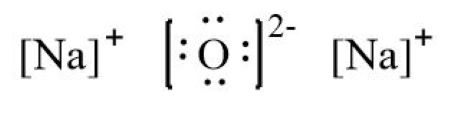
0 thoughts on “Sodium and oxygen lewis dot structure”