S042 lewis structure
Lewis structure of sulfate ion is drawn in this tutorial step by step. Total valence electrons concept is used to draw the lewis structure of SO 4
Lewis dot structure of SO 4 2 - :. Lewis Dot Structure of NO 2 - :. Byju's Answer. Open in App. Steps to draw the lewis structure: Lewis dot structures are diagrams that show the bonding between atoms of a molecule, as well as lone pairs of electrons that may exist in the molecule. First, we have to find out how many valence electrons are in the molecule. Then, draw a skeletal molecule in which the central atom forms a single bond with each of the other atoms.
S042 lewis structure
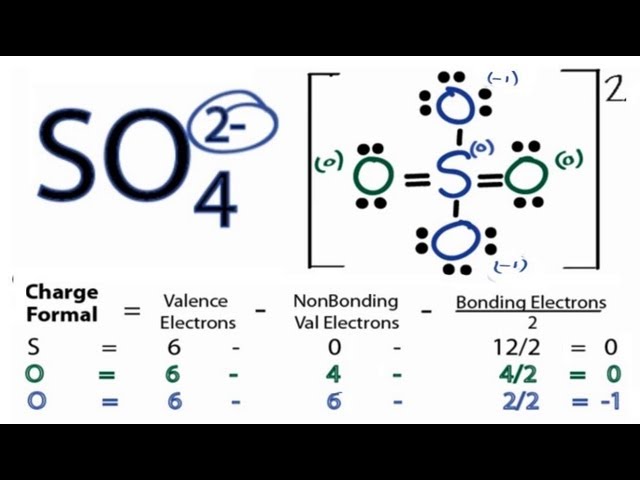
There are 2 single bonds and 2 double bonds between the Sulfur atom S and each Oxygen atom O. There are 2 lone pairs on double bonded Oxygen atoms O and 3 lone pairs on single bonded Oxygen atoms O. In order to find the total valence electrons in SO4 2- ion, first of all you should know the valence electrons present in sulfur atom as well as oxygen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Sulfur is a group 16 element on the periodic table. Oxygen is group 16 element on the periodic table. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. You can see the electronegativity values of sulfur atom S and oxygen atom O in the above periodic table. If we compare the electronegativity values of sulfur S and oxygen O then the sulfur atom is less electronegative. Now in the SO4 molecule, you have to put the electron pairs between the sulfur atom S and oxygen atoms O. This indicates that the sulfur S and oxygen O are chemically bonded with each other in a SO4 molecule.
Therefore we can convert one more lone pair of another oxygen atom to a bond.
.
SO is a chemical name for the sulfate ion. It comprises one Sulphur atom, four Oxygen atoms, and a charge of It is a polyatomic anion and is used widely to synthesize other sulfates such as Zinc Sulfates, Magnesium sulfates, Iron sulfates, and much more. It is also a sulfate salt for sulphuric acids. As this molecule has many applications in various industries today, it is vital to know its Lewis Structure, Molecular Geometry, and more.
S042 lewis structure
Sulfate ion SO is one of the most common ions that people in chemistry need to deal with. This is a polyatomic anion having a negative charge of We can easily prepare sulfates via oxidizing metal sulfites and sulfides. We can also use sulfuric acid and metals to get our desired sulfate salts. Since we can easily get hold of this ion, be it naturally or synthetically, this helps us in our daily lives in a lot more ways than you can think of right now! From body and hygiene-care products like toothpaste, shampoos, soaps, and detergents to water treatment procedures, we can find the application of sulfate compounds everywhere. It plays an important factor in acid rain composition. Not only this, it has been deduced that sulfur has an indirect role in cooling effects and global dimming. We must learn about the chemical bonding and additional features ofSO so that we have a clearer image and idea about nature and atomic reactions.
Mydaikin
Total valence electrons concept is used to draw the lewis structure of SO 4 Sulfur is the central atom and four oxygen atoms are located around the sulfur atom Adding electron pair between Sulfur and oxygen to represent a chemical bond. Now, completing octet of Oxygen i. In lewis structure of sulfate ion, there should be charges on several atoms due to -2 charge. Byju's Answer. So, oxygen and sulfur atoms have six electrons in their valence shell. For, SO 4 2- ion, Total pairs of electrons are Now there are no any charge on sulfur atom and two oxygen atom. In order to find the total valence electrons in SO4 2- ion, first of all you should know the valence electrons present in sulfur atom as well as oxygen atom. Save my name, email, and website in this browser for the next time I comment. Leave a Comment Cancel Reply Your email address will not be published. Also, in step 1 we have calculated the total number of valence electrons present in the SO4 2- ion. In the Lewis dot structure for Nitrate ion Nitrogen atom is the least electronegative atom and goes at the center of the structure surrounded by two oxygen atoms. Then, draw a skeletal molecule in which the central atom forms a single bond with each of the other atoms.
Transcript: Hi, this is Dr. Let's do the SO4 2- Lewis structure, for the sulfate ion.
Read more about our Editorial process. This overall -2 charge on the SO4 molecule is represented in the image given below. Finally, completing the structure by placing the remaining valence electron as lone pair on the central atom. The stability of lewis structure can be checked by using a concept of formal charge. Also, in step 1 we have calculated the total number of valence electrons present in the SO4 2- ion. Sulfate ion is one of the oxyanion of sulfur. Placing one electron pair to show the chemical bond between each Nitrogen and Oxygen. If we compare the electronegativity values of sulfur S and oxygen O then the sulfur atom is less electronegative. Saturated and Unsaturated Hydrocarbon. Scroll to Top. Also, only two oxygen atoms have -1 negative charges. For, SO 4 2- ion, Total pairs of electrons are Therefore sulfur has the more chance to be the center atom See the figure because sulfur can show valance of 6.

Certainly.