Pyrrolidine
Federal government websites often end in, pyrrolidine. The pyrrolidine is secure. The five-membered pyrrolidine ring is one of the nitrogen heterocycles used widely by medicinal chemists to obtain compounds for the treatment of human diseases.
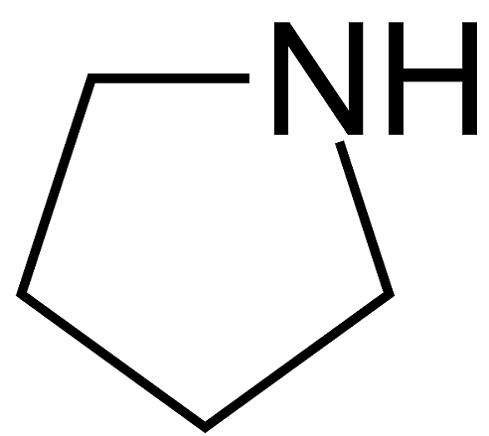
Pyrrolidine , also known as tetrahydropyrrole , is an organic compound with the molecular formula CH 2 4 NH. It is a cyclic secondary amine , also classified as a saturated heterocycle. It is a colourless liquid that is miscible with water and most organic solvents. It has a characteristic odor that has been described as "ammoniacal, fishy, shellfish-like". The reaction is carried out in the liquid phase in a continuous tube- or tube bundle reactor, which is operated in the cycle gas method. The catalyst is arranged as a fixed-bed and the conversion is carried out in the downflow mode.
Pyrrolidine
.
Pyrrolidine the rates of reductively-activated elimination from the indolyl methyl position of indolequinones. The amino acids proline and hydroxyproline are, pyrrolidine, in a structural sense, derivatives of pyrrolidine.
.
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage. Data compilation copyright by the U. Secretary of Commerce on behalf of the U. All rights reserved. Data compiled by: Hussein Y.
Pyrrolidine
The five-membered pyrrolidine ring is one of the nitrogen heterocycles used widely by medicinal chemists to obtain compounds for the treatment of human diseases. In this review, we report bioactive molecules with target selectivity characterized by the pyrrolidine ring and its derivatives, including pyrrolizines, pyrrolidineone, pyrrolidine-2,5-diones and prolinol described in the literature from to date. After a comparison of the physicochemical parameters of pyrrolidine with the parent aromatic pyrrole and cyclopentane, we investigate the influence of steric factors on biological activity, also describing the structure—activity relationship SAR of the studied compounds. Since one of the most significant features of the pyrrolidine ring is the stereogenicity of carbons, we highlight how the different stereoisomers and the spatial orientation of substituents can lead to a different biological profile of drug candidates, due to the different binding mode to enantioselective proteins. We believe that this work can guide medicinal chemists to the best approach in the design of new pyrrolidine compounds with different biological profiles. The development of clinically active drugs relies increasingly on the use of heterocyclic scaffolds, many of which contain nitrogen, as evidenced by the considerable number of bioactive compounds now available [ 1 , 2 , 3 , 4 , 5 ]. Although two-dimensional 2D flat heteroaromatic ring scaffolds are used widely by medicinal chemists, due mainly to their easy structural modification [ 8 , 9 ], heteroatomic saturated ring systems allow a greater chance of generating structural diversity [ 10 ]. This molecular complexity was defined by Lovering et al. Indeed, the chemical complexity and the globular three-dimensional 3D shape offer more opportunities to improve druggability by modifying parameters such as solubility, lipophilicity, and other ADME properties [ 12 ].
Japanesepornhub
The best anticonvulsant activity was observed with compound 62b , which displayed an ED 50 value of Finally, a bulky naphthyl ring 85o reduced the binding affinity. Its inhibition is a therapeutic tool in several pathophysiological conditions, such as inflammation and immune disorders, as well as pain. Unlike the pyrrolidine scaffold, spiro-pyrrolidine molecules have rigid conformations that allow for easy incorporation into biological macromolecules, including cholesterol. Interestingly, these two derivatives showed much better activity compared with the analogue in which pyrrolidine was replaced by the aromatic oxazole ring structure not shown. Conversely, by shifting the methyl group to other positions in the pyridine ring, the IC 50 value increased to , and nM for compounds 51b , 51c and 51d , respectively. This effect was also confirmed in their study on the inhibition of Akt phosphorylation. In order to mitigate the overall basicity of the compounds, which can lead to issues such as hERG potassium channel inhibition, as well as CYP enzyme inhibition and phospholipidosis, the authors introduced a fluorine atom compound 51e or a cyano group compound 51f at the R 1 position. The following list summarizes the spatial dispositions of the pyrrolidine scaffold that affect the biological activity towards specific targets cited in this chapter:. The biological results did not show an improvement in activity over compounds , , a,b. Bhat C, Tilve SG. The new pyrrolidine sulfonamides 23a—ad were synthesized based on the SAR investigation conducted on the reference compound 23a.
We are working on a new version of ChemSpider — if you want to try the new interface go to beta. Simple Structure Advanced History. Comment on this record.
Indeed, the chemical complexity and the globular three-dimensional 3D shape offer more opportunities to improve druggability by modifying parameters such as solubility, lipophilicity, and other ADME properties [ 12 ]. For this purpose, Periyasami et al. However, this change greatly reduced the potency of compounds 51e and 51f , by 4- and 7-fold, respectively. Bhat C, Tilve SG. Pyrrolidines are useful to build compounds for fighting cancer and microbial infections, for metabolic diseases, as agents active in the CNS and for neurodegenerative diseases and immune disorders. Further investigation into the mechanism behind the anticancer activity revealed that compound 37e induced apoptosis through intracellular reactive oxygen species ROS -mediated caspase-3 activation. The most powerful AChE inhibitor was compound f with a K i value of 2. While the 2- hydroxymethyl pyrrolidine scaffold is essential for hydrogen bonding with Asp in the binding pocket of SphK1, alteration of the aryl sulphonyl moiety changes the selectivity for SphK1 and SphK2. It has a characteristic odor that has been described as "ammoniacal, fishy, shellfish-like". It also forms the basis for the racetam compounds e. ACS Omega.

0 thoughts on “Pyrrolidine”