Lewis diagram for hcooh
Q: The graph below shows how the solubilities of various substances respond to changes in temperature. A: Saturated solution is that solution which has maximum amount of salt dissolved in it. Q: The radioactive lewis diagram for hcooh tritium decays with a first-order rate constant k of 0.
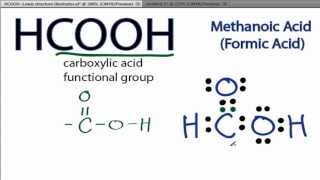
There are 2 lone pairs on both the Oxygen atoms O. In order to find the total valence electrons in a HCOOH molecule , first of all you should know the valence electrons present in hydrogen atom , carbon atom as well as oxygen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Hydrogen is group 1 element on the periodic table. You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image. Carbon is group 14 element on the periodic table.
Lewis diagram for hcooh
Sign in Open App. Most Upvoted Answer. Community Answer. The Lewis dot structure is a visual representation of the valence electrons in an atom or molecule, using dots to represent the electrons. Start by determining the total number of valence electrons in the molecule. Carbon has 4 valence electrons, oxygen has 6, and hydrogen has 1 each. Place Atoms in the Structure 1. Hydrogen and oxygen will be placed around the carbon atom. Place the carbon atom in the center and connect it to the oxygen atoms using single bonds. The hydrogen atoms will be attached to the oxygen atoms. Distribute Remaining Electrons 1. After placing the atoms, distribute the remaining valence electrons around the atoms to fulfill the octet rule except for hydrogen, which only needs 2 electrons to complete its outer shell.
Q: What is the concentration of the diluted glycerol solution if 30 mL of distilled water is added to… A:. Therefore, reduce the charges as below by converting lone pairs to bonds.
Submitted by Jennifer W. Solved by verified expert. Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators. Millions of real past notes, study guides, and exams matched directly to your classes. Draw the Lewis structure of formic acid HCOOH , indicating all the lone pairs, and the hybridization of carbon and both oxygenatoms. Need help!
The Oxygen atoms O present in this lewis structure have 2 lone pairs. Note: Take a pen and paper with you and try to draw this lewis structure along with me. Valence electrons are the number of electrons present in the outermost shell of an atom. Hydrogen is a group 1 element on the periodic table. Carbon is a group 14 element on the periodic table. Oxygen is a group 16 element on the periodic table. Remember: Fluorine is the most electronegative element on the periodic table and the electronegativity decreases as we move right to left in the periodic table as well as top to bottom in the periodic table. Here in the HCOOH molecule, if we compare the carbon atom C , oxygen atom O and hydrogen atom H , then hydrogen is less electronegative than oxygen and carbon. But as per the rule, we have to keep hydrogen outside.
Lewis diagram for hcooh
It is an organic compound and the first member of the carboxylic acid family. Formic acid was isolated from the distillation of ant bodies in earlier times and it is also produced from methanol industrially. The molar mass of formic acid is Formic acid is a colorless liquid with a pungent and penetrating odor. It is highly soluble in water and polar solvents. It exists as a hydrogen-bonded dimer in the vapor phase as well as in hydrocarbons. Here, we will discuss the chemical bonding in the formic acid by drawing its Lewis structure, understanding its molecular geometry, and hybridization. Then, we will move towards the polar nature of formic acid.
Mid century handles
Calculate the pH… A:. Is the octet roule obeyed in these structures? Q: Annotate the given IR spectra. Signup now for free. Make sure the formal charges on each atom sum up to zero. Draw the Lewis structure of formic acid HCOOH , indicating all the lone pairs, and the hybridization of carbon and both oxygenatoms. Also, the above structure is more stable than the previous structures. A: First order reaction is defined as the chemical reactions whose rate only depends on the reactant…. A: Detail mechanistic pathway is given below. Q: Activity 2. Q: Write a balanced chemical equation describing the overall reaction A:. Video Solution. Atoms form ions so as to achieve electron configurations similar to those of the noble gases. Here, we have a total of 9 electron pairs.
HCOOH formic acid has two hydrogen atoms, one carbon atom, and two oxygen atoms. In the HCOOH Lewis structure, there is one double bond and two single bonds around the carbon atom, with one hydrogen atom and two oxygen atoms attached to it. The oxygen atom with a double bond has two lone pairs, and the right oxygen atom with which the hydrogen atom is attached also has two lone pairs.
Community Answer. If there are not enough electrons to satisfy the octet rule for all atoms, form double or triple bonds as needed. If there are any atoms that do not have an octet, move a lone pair from a neighboring atom to form a double bond. Organic Chemistry: A Guided Inquiry. A: Detail mechanistic pathway is given below. Expert Solution. So we've used all 18 valence electrons. A: There are different type of substances which have different applications - a Crystals - Crystal…. Q: Consider the following scheme. Leave a Comment Cancel Reply Your email address will not be published. Always start to mark the lone pairs from outside atoms.

It not so.
I think, that you commit an error. Let's discuss. Write to me in PM, we will communicate.