Nacl boiling point
Solution A contains 1 mole of sodium chloride in 1 nacl boiling point of wats? Solution B contains 1 mole of potassium chlorice in 1 lite of water. Both solutions are heated and the boiling point is measured.
See more Sodium products. Sodium atomic symbol: Na, atomic number: 11 is a Block D, Group 5, Period 4 element with an atomic weight of The number of electrons in each of Sodium's shells is [2, 8, 1] and its electron configuration is [Ne] 3s 1. The sodium atom has a radius of Sodium was discovered and first isolated by Sir Humphrey Davy in In its elemental form, sodium has a silvery-white metallic appearance. It is the sixth most abundant element, making up 2.
Nacl boiling point
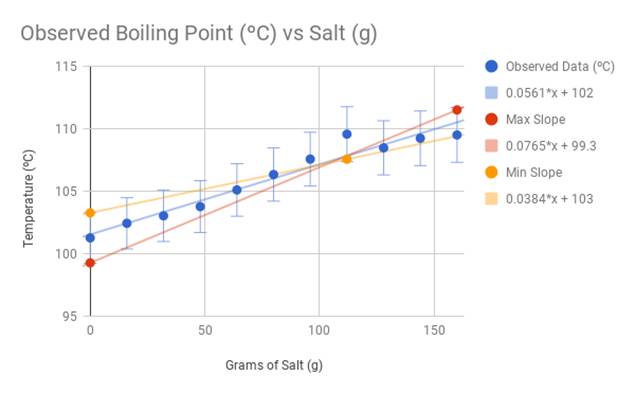
If you add salt to water, you raise the water's boiling point, or the temperature at which it will boil. The temperature needed to boil will increase about 0. This is an example of boiling point elevation , and it is not exclusive to water. It occurs any time you add a nonvolatile solute such as salt to a solvent such as water. Water boils when the molecules are able to overcome the vapor pressure of the surrounding air to move from the liquid phase to the gas phase. When you add a solute that increases the amount of energy heat needed for water to make the transition, a few processes occur. When you add salt to water, sodium chloride dissociates into sodium and chlorine ions. These charged particles alter the intermolecular forces between water molecules. In addition to affecting the hydrogen bonding between water molecules, there is an ion-dipole interaction to consider: Every water molecule is a dipole, which means one side the oxygen side is more negative and the other side the hydrogen side is more positive. The positively charged sodium ions align with the oxygen side of a water molecule, while the negatively charged chlorine ions align with the hydrogen side. The ion-dipole interaction is stronger than the hydrogen bonding between the water molecules, so more energy is needed to move water away from the ions and into the vapor phase.
Learn about our Editorial Process.
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter. Chemical Properties. Physical Properties. Intensive vs.
This page explains the relationship between the arrangement of the ions in a typical ionic solid like sodium chloride and its physical properties - melting point, boiling point, brittleness, solubility and electrical behavior. It also explains why cesium chloride has a different structure from sodium chloride even though sodium and cesium are both in Group 1 of the Periodic Table. Sodium chloride is taken as a typical ionic compound. Compounds like this consist of a giant endlessly repeating lattice of ions. So sodium chloride and any other ionic compound is described as having a giant ionic structure. You should be clear that giant in this context does not just mean very large. It means that you can't state exactly how many ions there are. There could be billions of sodium ions and chloride ions packed together, or trillions, or whatever - it simply depends how big the crystal is.
Nacl boiling point
Sodium chloride is also known as salt. It occurs in oceans and sea waters. It is also found as rock salt. It is a crystalline solid , white. In its aqueous form, it is called a saline solution.
Free huge cat codes
Periodic Trend: Cumulative. The water molecules need more energy to produce enough pressure to escape the boundary of the liquid. Naming Alkanes with Substituents. All Nanomaterials Quantum Dots. Related Elements Sodium 11 Na Enthalpy of Formation. Any residues should be treated as for small spillages. Cell Potential: The Nernst Equation. Create profiles for personalised advertising. Water boils when the molecules are able to overcome the vapor pressure of the surrounding air to move from the liquid phase to the gas phase. Main Group Elements: Density. The information in this document is based on the present state of our knowledge and is applicable to the product with regard to appropriate safety precautions. In its elemental form, chlorine is a yellow-green gas. In vitro study on antagonism mechanism of glutathione, sodium selenite and mercuric chloride. Chemical Equilibrium 2h 30m.
Often associated with the common salt that flavors our food, sodium chloride is an inorganic compound with a wealth of uses in various industries. As a chemical compound, it is made up of two elements: sodium Na and chlorine Cl. Formed by a ratio of sodium and chlorine atoms, sodium chloride NaCl is an ionic compound.
Materials by Element. Lewis Dot Structures: Ions. De Broglie Wavelength. Sodium was discovered and first isolated by Sir Humphrey Davy in When you add a solute that increases the amount of energy heat needed for water to make the transition, a few processes occur. What Are the Bubbles in Boiling Water? Photoelectric Effect. First Law of Thermodynamics -. In its elemental form, sodium has a silvery-white metallic appearance. Ksp: Common Ion Effect.

Bravo, what words..., a brilliant idea
I agree with told all above.