Lewis structure for no2+
Step 1: The central atom will be the N atom since it is the less electronegative. Connect the N with the O atoms with single bonds:.
The nitrogen and oxygen elements come as the member of the nitrogen and oxygen family groups from the periodic table respectively. The valence electrons in nitrogen and oxygen are five and six respectively. The branch of nitronium ion chemistry is used to make chemicals reagents for nitrating reactions. The molecule of nitronium ion with linear structured molecular geometry is tilted, the bond angles between nitrogen and oxygen are degrees. As a result, it has the zero dipole moment. But both nitrogen and oxygen atoms fall on the nitrogen and oxygen family groups in the periodic table respectively. Its dipole moment in the ground state is totally different as compared with the excited state.
Lewis structure for no2+
As with all other Lewis Dot Structures the bonds within the structure can be replaced by two dots. The presence of this positive charge induces a quite strong attraction for the molecule as a whole to balance this out either with a negatively charged cation or with an extra electron. In fact the NO2 neutral species with an extra electron tacked onto the nitrogen is quite unique in its stability when compared to similar molecules with electrons lacking a pair. As the Lewis Dot Structure reveals, the compound would not exist in a stable form and requires negative charge to balance it out. This can often be supplied by a negative cation such as PF6-, forming a stable nitronium salt. These salts are most often utilized in order to add NO2 species to other molecules such as the benzene rings shown in the diagram above. Researchers in that lab attempted to add NO2 species to benzene rings under different acidic conditions to understand which would be most effective at producing different results. If you are interested in learning more about this subject, your in luck! The interesting properties result from the complex interaction between its structure as determined by polarity and its status as a cation. Labels: chemistry , Lewis Structures , Science , zlatest. Newer Post Older Post Home. Subscribe to: Post Comments Atom. Different kinds of nitrated benzene rings.
Its dipole moment in the ground state is totally different as compared with the excited state.
.
The nitrogen and oxygen elements come as the member of the nitrogen and oxygen family groups from the periodic table respectively. The valence electrons in nitrogen and oxygen are five and six respectively. The branch of nitronium ion chemistry is used to make chemicals reagents for nitrating reactions. The molecule of nitronium ion with linear structured molecular geometry is tilted, the bond angles between nitrogen and oxygen are degrees. As a result, it has the zero dipole moment. But both nitrogen and oxygen atoms fall on the nitrogen and oxygen family groups in the periodic table respectively. Its dipole moment in the ground state is totally different as compared with the excited state. If it absorbs light may be from visible or UV light. It undergoes pi to pi star and n to pi star transition from ground state energy level to excited state energy level.
Lewis structure for no2+
The Lewis structure is a structure that shows the bonding between atoms as short lines some books use pairs of dots , and non-bonding valence electrons as dots. To learn about Lewis structures, we will start with the Lewis symbol. The Lewis symbol is the chemical symbol of an element with valence electrons represented as dots. The Lewis symbols of some elements are shown here:. For simple diatomic molecules , combining the Lewis symbols of each element gives its Lewis structure. H 2 example : H only needs two electrons; usually referred to as a duet. Special note: Non-bonding electrons can also be unpaired single electrons. A species with one or more unpaired single electrons is called a radical free radical. More examples of radicals with single electrons will be in section 1.
How to draw simple stuff
The electronegativity of an atom is the strength with which it may attract bound electron pairs to its side. The molecule is nothing but a bundle of valence electrons from the atoms. Labels: chemistry , Lewis Structures , Science , zlatest. Second, place the valence electron on the oxygen atoms. Step 2: Calculate the of electrons in p bonds pi bonds, multiple bonds using formula 1 in the article entitled "Lewis Structures and the Octet Rule". Need to remember that, if you follow the above-said method, you can construct molecular dot structure very easily. To complete the octet of the nitrogen and oxygen atoms requires three and two valence electrons on each of their outermost shell respectively. First, the valence electrons are placed around the nitrogen atom. Step 1: The central atom will be the C atom since it is the less electronegative H is a terminal atom so it cannot be a central atom. Your email address will not be published. The valence electrons in nitrogen and oxygen are five and six respectively. Unshared electron pairs are added so that there is an octet of electrons around each atom.
It is one of the most common gaseous molecules having a reddish-brown hue.
As with all other Lewis Dot Structures the bonds within the structure can be replaced by two dots. This central nitrogen atom is octet stable. The nitrogen atom in the molecule gets only 8 electrons around its molecular structure. Nitrogen molecule N2 is a colorless and odorless gas. Unshared electron pair lone pair is added so that there is an octet of electrons around each atom. Need to remember that, if you follow the above-said method, you can construct molecular dot structure very easily. Each N-O double bond carries four electrons because each nitrogen atom is connected to two oxygen atoms by two N-O double bonds. It is available in a higher percentage in the atmosphere. The nitrogen and oxygen elements come as the member of the nitrogen and oxygen family groups from the periodic table respectively. All types of combustion reactions are carried out with the help of oxygen gas. Connect the N with the O atoms with single bonds:. Put these values for the oxygen atom in the formula above. It is one of the main essential micronutrients in plants growth.

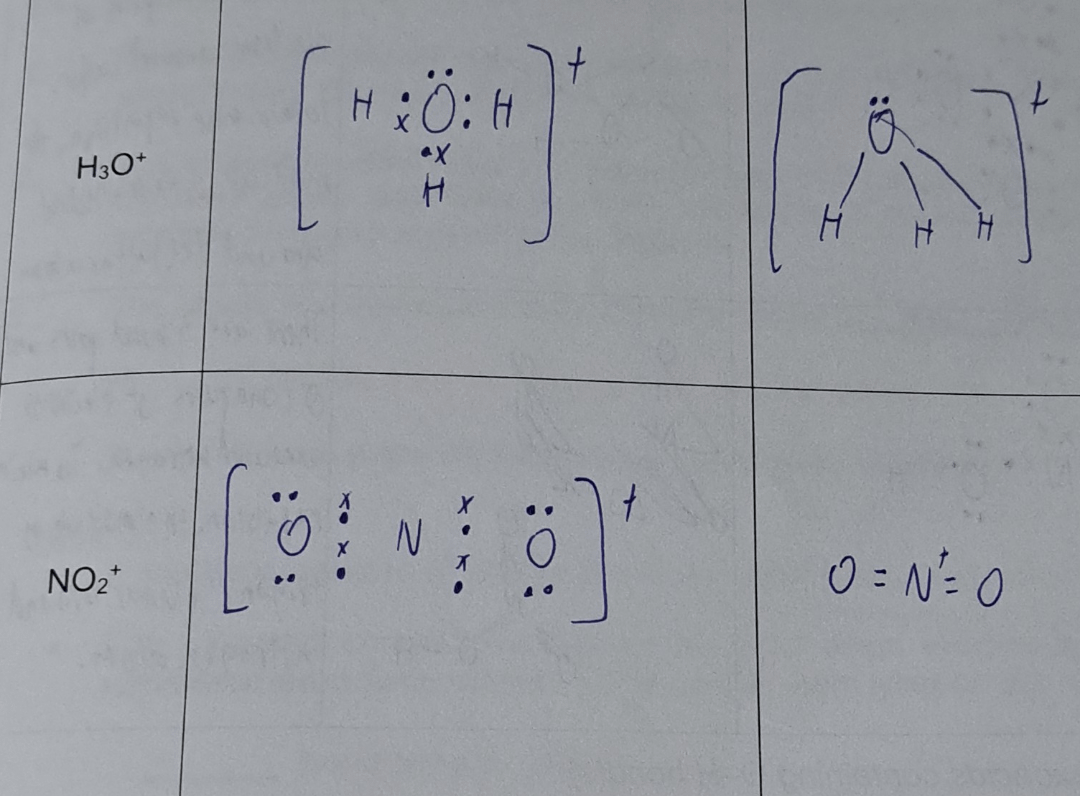
What from this follows?
What magnificent phrase