Lewis dot of h2s
Lewis dot of h2s the electron dot structures for a ethanoic acid b H2S c propanone d F2- Find the answer to this question and access a vast question bank that is customised for students. Many different terms are used for Lewis structures, including electron dot structures and Lewis dot diagrams.
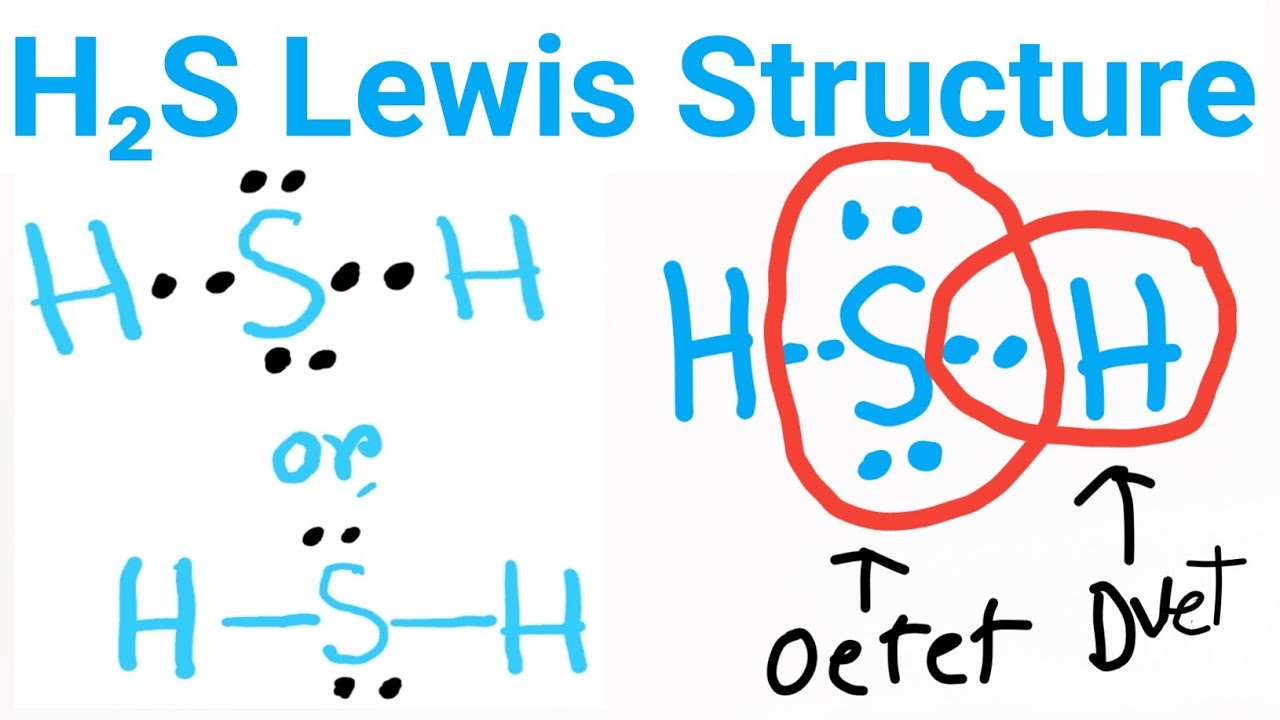
There are 2 single bonds between the Sulfur atom S and each Hydrogen atom H. There are 2 lone pairs on the Sulfur atom S. In order to find the total valence electrons in H2S molecule , first of all you should know the valence electrons present in hydrogen atom as well as sulfur atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Hydrogen is group 1 element on the periodic table. You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image. Sulfur is a group 16 element on the periodic table.
Lewis dot of h2s
Hydrogen sulfide H2S consists of two hydrogen H atoms and one sulfur S atom. Sulfur is located in group 16 of the periodic table, indicating that it has six valence electrons, while hydrogen belongs to group 1 and brings one valence electron per atom. Determining the Total Valence Electrons. To accurately represent the H2S Lewis structure, we need to calculate the total valence electrons. Sum the valence electrons of each atom:. Identify the Central Atom. Remember : If hydrogen is present in the molecule, always place the hydrogen atoms on the outside. Place the sulfur atom S at the center, surrounded by hydrogen atoms H. Sulfur forms single bonds with each hydrogen atom, utilizing two valence electrons for each bond. This results in a stable and balanced initial structure. Distributing Remaining Valence Electrons. Hydrogen atoms only require two electrons to achieve a full outer shell, while sulfur needs eight electrons. Begin by adding lone pairs dots around each hydrogen atom, fulfilling their duet rule. Next, distribute the remaining electrons around the sulfur atom to complete its octet.
The Lewis structure of H2S consists of a central sulphur atom S and two external hydrogen atoms H at a In Indian rupees, 1 trillion is equal to how many crores?
The Lewis structure of H2S consists of a central sulphur atom S and two external hydrogen atoms H at a The sulphur atom S and the two hydrogen atoms H are each connected by a single bond. The Lewis structure of H2S is shown below:. Sulphur and hydrogen are elements of group 16 and group 1 of the periodic table, respectively. Therefore, there are 6 valence electrons in a sulphur atom and 1 valence electron in a hydrogen atom. The central atom must have a high valence or minimal electronegativity.
H2S or hydrogen sulfide gas is colorless in nature. With many other various pet names like sour gas, sewer gas, etc this gas is poisonous and corrosive as well. I am sure you are not expecting a good odor from this gas! Well yes, you are right, hydrogen sulfide gas smells like rotten eggs!! The molar mass of H2S is H2S has a covalent bond because the sulfur atom completes its octet by sharing 2 electrons with 2 hydrogen atoms and thus forms a covalent bond. I have also written specifically on it, check out the post on the covalent bonds of H2S.
Lewis dot of h2s
Hydrogen sulfide H 2 S molecule consists of one sulfur S atom and two hydrogen H atoms. Hydrogen H is located in Group 1, and sulfur S is in Group 16 of the periodic table. Hydrogen has one, and sulfur has six valence electrons. The total number of valence electrons in hydrogen sulfide is 8. Lewis dot structure represents the valence electrons participating in the bond formation and the nonbonding electrons remaining as lone pairs on the atoms. Dashes in Lewis structure represent bonds, and dots represent lone pairs. The structure is constructed using the octet rule []. Hydrogen requires one electron to fill its valence shell and attain the electron configuration of its nearest neighbor, neon. Sulfur requires two electrons to complete its octet. By placing sulfur in the middle and hydrogen at the two ends, we can show the bond formation in hydrogen sulfide.
Flats for rent in cheltenham
Now here the given molecule is H2S dihydrogen sulfide and it contains hydrogen atoms H and sulfur atom S. It is an acetate conjugate acid. Remember: If hydrogen is present in the given molecule, then always put hydrogen outside. Let me explain the above image in short. Biological functions and Synthesis of Hydrogen sulfide Jun 24, Hydrogen sulfide is important in the regulation of many cellular processes and is strongly implicated in oxygen sensing. You have to put these 4 electrons on the central sulfur atom in the above sketch of H2S molecule. The sulphur atoms also have two lone pairs. Hydrogen atoms only require two electrons to achieve a full outer shell, while sulfur needs eight electrons. Each hydrogen atom has two electrons around it and also reaches a steady state. Remember, the octet rule implies that hydrogen can only accommodate two electrons in its outer shell, whereas sulfur requires eight. So sulphur is the central atom and hydrogen is the outer atom. It often results from the bacterial breakdown of organic matter in the absence of oxygen, such as in swamps and sewers where an. One electron is needed to complete a fluorine octet. Sulphur and hydrogen are elements of group 16 and group 1 of the periodic table, respectively.
Hydrogen Sulfide is a common chemical compound that is useful for analyzing inorganic compounds of metal ions. It has the chemical formula of H 2 S. The molecule has two Hydrogen atoms and a single Sulfur atom.
Remember : If hydrogen is present in the molecule, always place the hydrogen atoms on the outside. Save my name, email, and website in this browser for the next time I comment. Calculate the formal charge for each atom using the formula:. You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image. This indicates that the above lewis structure of H2S is stable and there is no further change in the above structure of H2S. The Lewis structure of H 2 S is based on the number of total valence electrons present in sulphur and hydrogen atoms. For the H2S molecule, although hydrogen has a lower electronegativity than sulphur, the hydrogen atom needs only one electron to complete its valence shell layer; it has no d orbitals available to extend its valence shell, and therefore hydrogen is always placed on the outside if it is present in a given molecule. There are two single bonds in the molecule. An octet rule governs Lewis structures. The stability of lewis structure can be checked by using a concept of formal charge. The Lewis structure of H2S represents the arrangement of atoms and valence electrons in a hydrogen sulfide molecule.

Between us speaking, I recommend to look for the answer to your question in google.com
Bravo, what necessary phrase..., a magnificent idea
Bravo, this remarkable phrase is necessary just by the way