Ionic compounds are soluble in water
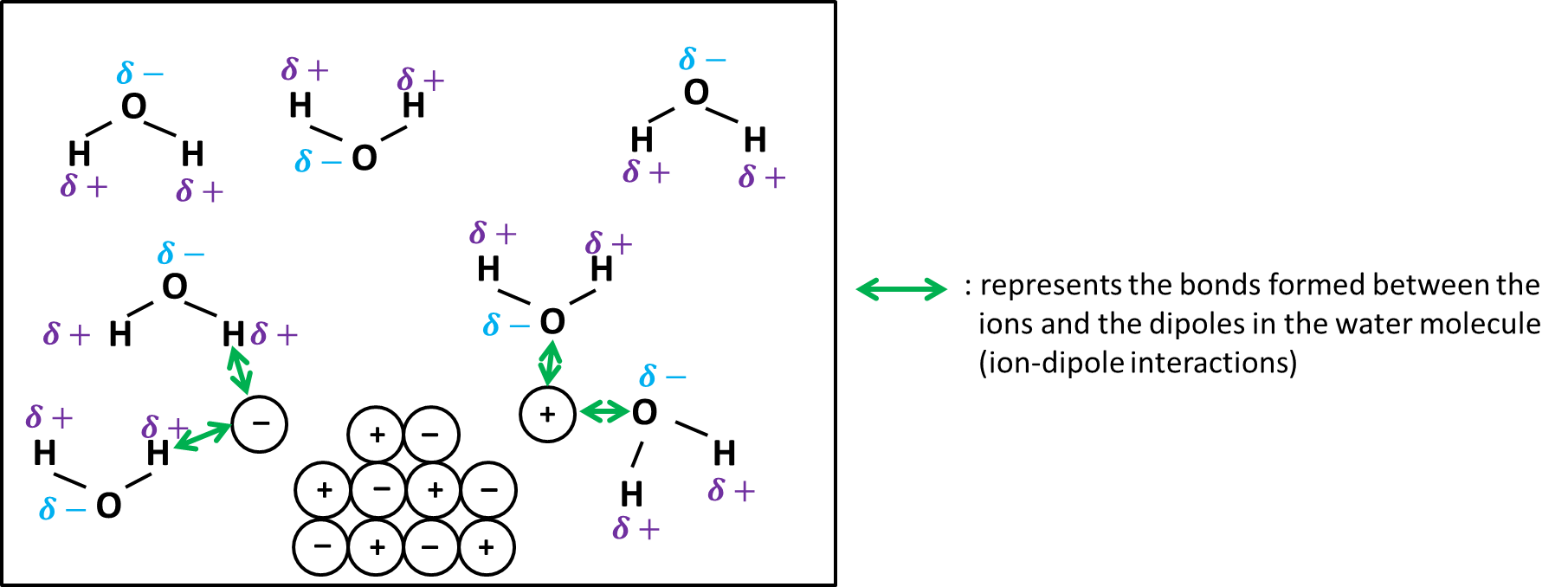
To dissolve an ionic compound, the water molecules must be able to stabilize ionic compounds are soluble in water ions that result from breaking the ionic bond. The "O" atom has a partial negative charge, and the "H" atoms have a partial positive charge. When you place an ionic substance in water, the water molecules attract the positive and negative ions from the crystal. The positive ions have several water molecules around them, all with their "O" atoms close to the positive ion.
We have learned that solutions can be formed in a variety of combinations using solids, liquids, and gases. We also know that solutions have constant composition, and that this composition can be varied up to a point to maintain the homogeneous nature of the solution. But how exactly do solutions form? Why is it that oil and water will not form a solution, and yet vinegar and water will? Why could we dissolve table salt in water, but not in vegetable oil? The reasons why solutions will form will be explored in this section, along with a discussion of why water is used most frequently to dissolve substances of various types. In most cases, only a certain maximum amount of solute can be dissolved in a given amount of solvent.
Ionic compounds are soluble in water
The extent to which a substance may be dissolved in water, or any solvent, is quantitatively expressed as its solubility , defined as the maximum concentration of a substance that can be achieved under specified conditions. Substances with relatively large solubilities are said to be soluble. A substance will precipitate when solution conditions are such that its concentration exceeds its solubility. Substances with relatively low solubilities are said to be insoluble , and these are the substances that readily precipitate from solution. More information on these important concepts is provided in the text chapter on solutions. Therefore, Pb NO 3 2 is soluble. Solubility is the maximum amount of solute that can dissolve in specific amount of solvent. Not all ionic compounds are able soluble in water. We use the solubility rules to predict whether an ionic compound dissolves in water or not. Search site Search Search. Go back to previous article. Sign in.
Feb 25, Moving to insoluble compounds, sulfides and hydroxides are insoluble, with the exception of their salts with alkali metals and barium. Chemists have formulated a set of empirical guidelines to predict the solubility of ionic compounds in water.
A subscription to JoVE is required to view this content. We recommend downloading the newest version of Flash here, but we support all versions 10 and above. If that doesn't help, please let us know. Unable to load video. Please check your Internet connection and reload this page.
Ionic compounds are a type of chemical compound that consists of positively charged ions cations and negatively charged ions anions held together by electrostatic forces. One important property of ionic compounds is their solubility, which refers to the ability of a compound to dissolve in a solvent. Solubility is influenced by various factors, including the nature of the ions, the polarity of the solvent, and the temperature. Some ionic compounds are highly soluble in water, while others are insoluble. Understanding the solubility of different ionic compounds is crucial in various fields, such as chemistry, medicine, and environmental science.
Ionic compounds are soluble in water
Chemistry sounds arcane, but the answers to many of its questions can be found in everyday life. When we ponder whether ionic compounds are soluble in water, we can simply think about table salt. Table salt is sodium chloride , an ionic compound comprising sodium cations and chloride anions. From experience, we know that it dissolves quickly in water, adding flavours to our porridge, soup, and sauce. Water molecules can attract most ions very well, luring the ions away from each other. This allows the ionic compound to dissolve, whereby water molecules and ions intermingle and mix thoroughly. In water, the electrostatic forces of attraction between oppositely charged ions are overcome, allowing the ions to dissociate and dissolve.
Enthusiastic meaning
The diagram shows eight purple spheres labeled K superscript plus and eight green spheres labeled C l superscript minus mixed and touching near the center of the diagram. A simple water-soluble ionic compound like sodium chloride dissolves in water by breaking up into monatomic ions. If the hydration energy of the compound is lesser than the lattice energy, the compound will not dissolve. Most familiar is the conduction of electricity through metallic wires, in which case the mobile, charged entities are electrons. The figure below illustrates the above process and shows the distinction between unsaturated and saturated. Ionic compounds dissolve in polar solvents, especially water. Get cutting-edge science videos from J o VE sent straight to your inbox every month. The sugar we use to sweeten coffee or tea is a molecular solid , in which the individual molecules are held together by relatively weak intermolecular forces. The solubility of a solute is its maximum possible concentration at solubility equilibrium in a given amount of solvent. When the maximum amount of solute has been dissolved in a given amount of solvent, we say that the solution is saturated with solute. Two of the green C l superscript minus spheres are surrounded by three of the red and white clusters, with the red spheres closer to the green spheres than the white spheres. A solution of
When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. These substances constitute an important class of compounds called electrolytes. Substances that do not yield ions when dissolved are called nonelectrolytes.
In the case of insoluble salts, the strong interionic forces that bind the ions in the solid are stronger than the ion-dipole forces between individual ions and water molecules. Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. Please check your Internet connection and reload this page. What is the chemical formula for the ionic compound formed when elements of Ca and N react? Water molecules in front of and behind the ions are not shown. Summary Solubility is the specific amount of solute that can dissolve in a given amount of solvent. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the strong electrostatic forces between them. Ionic compounds dissolve in water due to the difference between its lattice energy and its hydration energy. Water is a polar molecule. You have unlocked a 2-hour free trial now. Chapter Electrochemistry. The reasons why solutions will form will be explored in this section, along with a discussion of why water is used most frequently to dissolve substances of various types. Embed Languages Share. We have learned that solutions can be formed in a variety of combinations using solids, liquids, and gases.

I congratulate, what words..., a brilliant idea
How will order to understand?
You have hit the mark. It seems to me it is excellent thought. I agree with you.