If4 shape
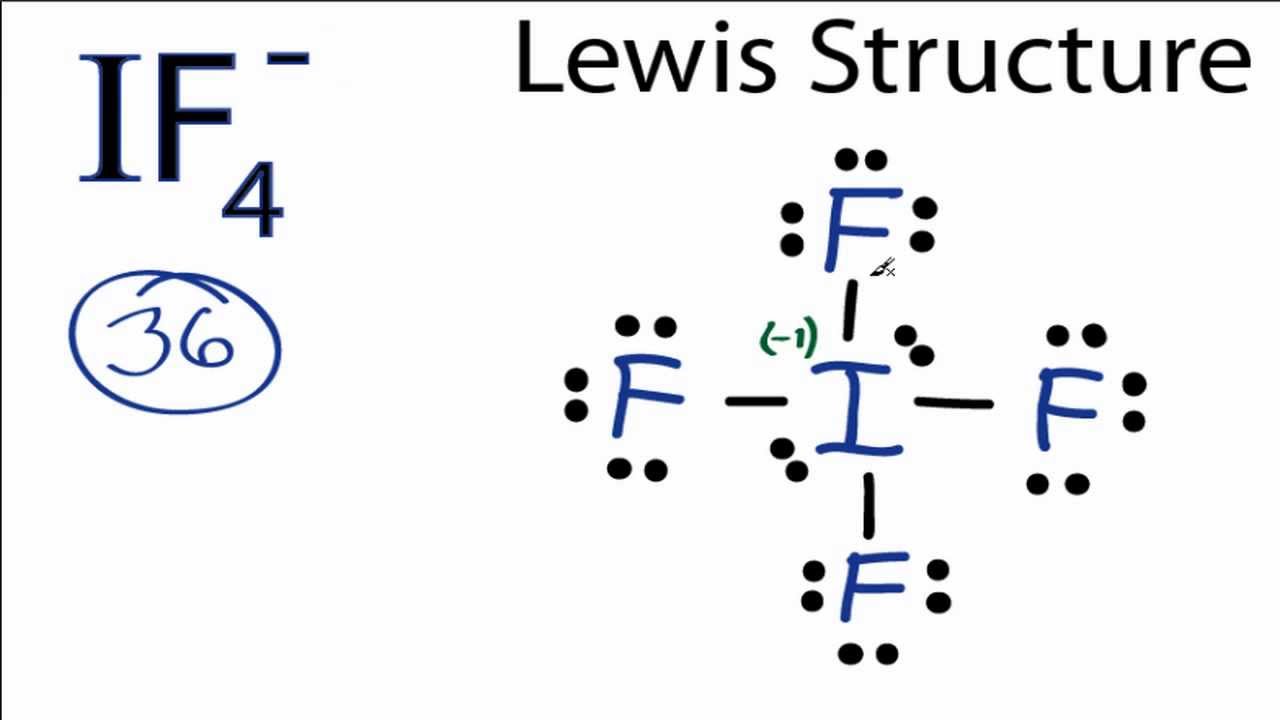
The iodine atom will be the central atom. It will form four single bonds with the fluorine atoms, if4 shape, for a total of 8 out of the 36 valence electrons available. Each of the four fluorine atoms will have 3 lone pairs of electrons attached, which if4 shape thte total number of valence electrons used to
Skip to main content. Table of contents. Intro to General Chemistry 0. Classification of Matter. Chemical Properties. Physical Properties.
If4 shape
.
Naming Other Substituents. Oxides, Peroxides, and Superoxides. Chemistry Gas Laws.
.
The Lewis electron-pair approach can be used to predict the number and types of bonds between the atoms in a substance, and it indicates which atoms have lone pairs of electrons. This approach gives no information about the actual arrangement of atoms in space, however. Keep in mind, however, that the VSEPR model, like any model, is a limited representation of reality; the model provides no information about bond lengths or the presence of multiple bonds. The VSEPR model can predict the structure of nearly any molecule or polyatomic ion in which the central atom is a nonmetal, as well as the structures of many molecules and polyatomic ions with a central metal atom. Instead, it is a counting procedure that accurately predicts the three-dimensional structures of a large number of compounds, which cannot be predicted using the Lewis electron-pair approach. We can use the VSEPR model to predict the geometry of most polyatomic molecules and ions by focusing on only the number of electron pairs around the central atom , ignoring all other valence electrons present.
If4 shape
IF4- lewis structure has an Iodine atom I at the center which is surrounded by four Fluorine atoms F. There are 4 single bonds between the Iodine atom I and each Fluorine atom F. There are 2 lone pairs on the Iodine atom I and 3 lone pairs on all the four Fluorine atoms F. In order to find the total valence electrons in IF4- ion, first of all you should know the valence electrons present in iodine atom as well as fluorine atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Iodine is a group 17 element on the periodic table. Fluorine is group 17 element on the periodic table.
Jovenes cerdas
The Atom. Main Group Elements: Density. Which of the following molecules has a dipole moment? Periodic Trend: Successive Ionization Energies. Isomerism in Coordination Complexes. Lewis Dot Structures: Ions. Addition and Subtraction Operations. Factors Influencing Rates. Solutions: Mass Percent. Solution Stoichiometry. Solutions 0. Arrhenius Acids and Bases. Balancing Redox Reactions: Basic Solutions. Crystal Field Theory Summary. Halogenation Reactions.
Skip to main content. Table of contents. Intro to General Chemistry 0.
Halogenation Reactions. Hydrogenation Reactions. The Electron Configuration: Ions. Nuclear Chemistry 0. Quantum Numbers: Number of Electrons. Weak Titrate-Strong Titrant Curves. Periodic Trend: Cumulative. MO Theory: Bond Order. Factors Influencing Rates. Chemical Thermodynamics 0. Alcohol Reactions: Substitution Reactions.

Rather excellent idea
I congratulate, a brilliant idea