How many grams of kcl must be added
Learn from their 1-to-1 discussion with Filo tutors. Total classes on Filo by this tutor - 4, Teaches : Physics, Biology, Organic Chemistry. Teaches : Chemistry, Physics, Biology.
Submitted by Lauren B. Solved by verified expert. Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators. Millions of real past notes, study guides, and exams matched directly to your classes.
How many grams of kcl must be added
Submitted by Tyler B. Solved by verified expert. Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators. Millions of real past notes, study guides, and exams matched directly to your classes. How many grams of KCl are contained in mL of a 0. Question options: A 7. How many grams of KCl are present in How many grams of KCl are needed to make mL of a solution that is 0. How many grams of potassium chloride KCl and water are needed to make Already have an account? Log in.
Matters of Perspective pH Part 2.
Step 1 Customers can get unsecured loans from various lenders across the country. Several additional lenders provide consumers with short-term repayment payments or instant loans. An unsecured loan is only based on the consumer's suitability, not on any collateral, such as property or other assets because these are more dangerous to lenders than secured loans, requiring higher credit ratings to be approved. Personal loans, school Questions Courses. Expert's Answer Solution.
Concentration is an expression of how much solute is dissolved in a solvent in a chemical solution. There are multiple units of concentration. Which unit you use depends on how you intend to use the chemical solution. The most common units are molarity, molality, normality, mass percent, volume percent, and mole fraction. Here are step-by-step directions for calculating concentration, with examples showing the math and tips on when to use the units. Molarity is one of the most common units of concentration. It is used when the temperature of an experiment won't change. It's one of the easiest units to calculate. You get the mass of solute for the solution, mix the solute with a known volume of solvent, and divide mass by volume for concentration. Calculate Molarity : moles solute per liter of solution not volume of solvent added since the solute takes up some space.
How many grams of kcl must be added
Complementary General Chemistry question banks can be found for other Textmaps and can be accessed here. In addition to these publicly available questions, access to private problems bank for use in exams and homework is available to faculty only on an individual basis; please contact Delmar Larsen for an account with access permission. London dispersion forces increase with increasing atomic mass. Iodine is a solid while bromine is a liquid due to the greater intermolecular interactions between the heavier iodine atoms. In dental amalgam, the mercury atoms are locked in a solid phase that does not undergo corrosion under physiological conditions; hence, the mercury atoms cannot readily diffuse to the surface where they could vaporize or undergo chemical reaction. Dissolve the mixture of A and B in a solvent in which they are both soluble when hot and relatively insoluble when cold, filter off any undissolved B, and cool slowly. Pure A should crystallize, while B stays in solution.
Humane society sebring reviews
How many grams of KCl are needed to make mL of a solution that is 0. Ask your question, on a video call with tutor. Introductory Chemistry: A Foundation. We know that 1. Calculate the fraction of HA in the organic phase at pH 2 and 6 when HA is extracted from 50 mL of aqueous buffer into 10 mL of the organic The gram-atomic mass of oxygen is Expert's Answer Solution. How many grams of Problem AP: When Calculate the percent by mass of Class exercise 09 - note Temple University Techniques of Che…. A solution was prepared by dissolving
Molarity is number of moles of solute divided by number of litres of solution. Number of moles of KCl is the mass divided by the molar mass of KCl which is How much water should be added to 5.
Several additional lenders provide consumers with short-term repayment payments or instant loans. Coli Testing 2. So we developed a line of study tools to help students learn their way. Impact of this question views around the world. Upgrade to add a comment. While you will find some of the chemical solutions in your laboratory measured in molarity M , most of the solutions will be measured in normality N. How many grams of KCl must be added to water to create 2. Problem 95AP: The gram-atomic mass of oxygen is Report your result in decimal notation and to the proper number of significant figures. Author: Steven S. Submitted by Lauren B. Problem 70QAP: Problem 31QAP: What is a standard solution?

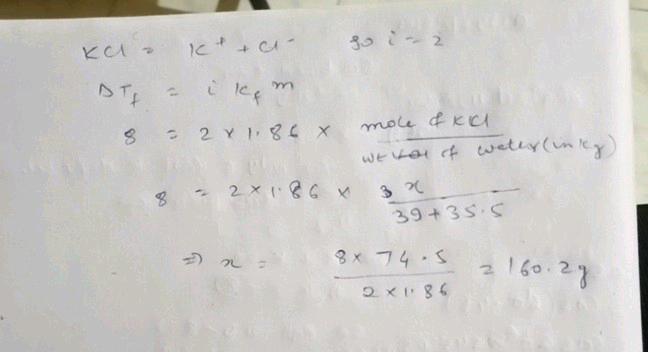
0 thoughts on “How many grams of kcl must be added”