Exocytosis
Thank you for visiting nature.
Federal government websites often end in. The site is secure. Costanzo 4, Perugia, Italy; ti. Beyond the consolidated role in degrading and recycling cellular waste, the autophagic- and endo-lysosomal systems play a crucial role in extracellular release pathways. Lysosomal exocytosis is a process leading to the secretion of lysosomal content upon lysosome fusion with plasma membrane and is an important mechanism of cellular clearance, necessary to maintain cell fitness. Exosomes are a class of extracellular vesicles originating from the inward budding of the membrane of late endosomes, which may not fuse with lysosomes but be released extracellularly upon exocytosis. In addition to garbage disposal tools, they are now considered a cell-to-cell communication mechanism.
Exocytosis
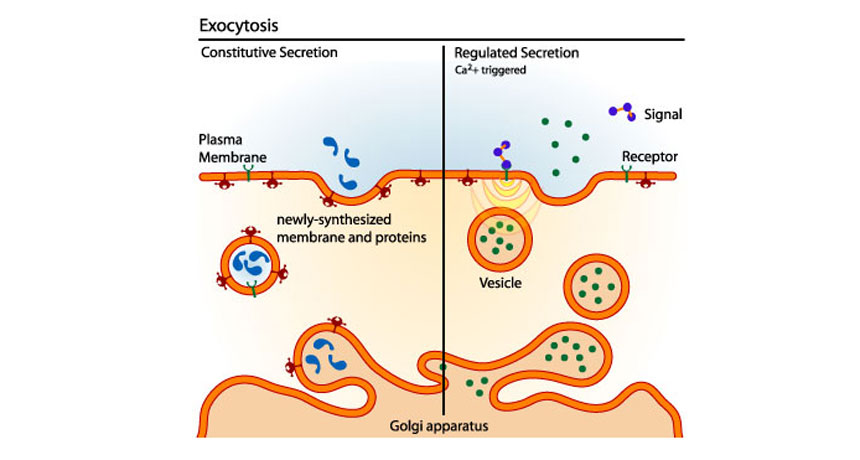
Exocytosis is the process of moving materials from within a cell to the exterior of the cell. This process requires energy and is therefore a type of active transport. Exocytosis is an important process of plant and animal cells as it performs the opposite function of endocytosis. In endocytosis, substances that are external to a cell are brought into the cell. In exocytosis, membrane-bound vesicles containing cellular molecules are transported to the cell membrane. The vesicles fuse with the cell membrane and expel their contents to the exterior of the cell. The process of exocytosis can be summarized in a few steps. Exocytosis serves several important functions as it allows cells to secrete waste substances and molecules, such as hormones and proteins. Exocytosis is also important for chemical signal messaging and cell to cell communication. In addition, exocytosis is used to rebuild the cell membrane by fusing lipids and proteins removed through endocytosis back into the membrane. Exocytotic vesicles containing protein products are typically derived from an organelle called the Golgi apparatus , or Golgi complex. Proteins and lipids synthesized in the endoplasmic reticulum are sent to Golgi complexes for modification and sorting. Once processed, the products are contained within secretory vesicles, which bud from the trans face of the Golgi apparatus.
In this context, an impairment of exosome biogenesis and secretion in neurodegenerative exocytosis caused by genetic mutations has been demonstrated [ 66 ], exocytosis. Nanoparticle analysis sheds budding insights into genetic drivers of extracellular vesicle biogenesis.
As an active transport mechanism, exocytosis requires the use of energy to transport material. Exocytosis and its counterpart, endocytosis , are used by all cells because most chemical substances important to them are large polar molecules that cannot pass through the hydrophobic portion of the cell membrane by passive means. Exocytosis is the process by which a large amount of molecules are released; thus it is a form of bulk transport. Exocytosis occurs via secretory portals at the cell plasma membrane called porosomes. Porosomes are permanent cup-shaped lipoprotein structure at the cell plasma membrane, where secretory vesicles transiently dock and fuse to release intra-vesicular contents from the cell. In exocytosis, membrane-bound secretory vesicles are carried to the cell membrane , where they dock and fuse at porosomes and their contents i.
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Search for courses, skills, and videos. Membrane transport. About About this video Transcript. Exocytosis is a form of bulk transport during which large numbers of molecules are transported out of the cell. In exocytosis, a vesicle a small, membrane-bound compartment containing the molecules to be released fuses with the cell membrane, and the contents of the vesicle are expelled.
Exocytosis
Exocytosis is the natural process of transporting molecules from within a cell to the outside space. In this process, the vesicles containing the fluid enclosed by a lipid bilayer fuse with the plasma membrane to release their contents outside the cell. It occurs in all living cells, from invertebrates and protozoa to plants and human. The basic process starts when a membrane-bound vesicle called secretory vesicle is transported from inside the cell to the cell membrane. The vesicle then transiently fuses with the cell membrane and eventually releases its content outside the cell. Large and complex protein molecules like enzymes, peptide hormones, and antibiotics are regularly released through this energy-dependent process. Step 1: Vesicle Trafficking : The first step of exocytosis during which the secretory vesicle move from their spot of creation to the cell membrane. This is an energy-consuming step. Step 2: Vesicle Tethering : On reaching the cell membrane, the outgoing vesicle becomes linked to, and is pulled into close contact with the cell membrane. Step 3: Vesicle Docking : The vesicle then gets transiently attached to the cell membrane, with its own membrane beginning to merge with the latter.
Ark tusoteuthis
We find that during exocytosis, the distal silica deposition vesicle membrane and the plasma membrane gradually detach from the mineral and disintegrate in the extracellular space, without any noticeable endocytic retrieval or extracellular repurposing. Schulz, D. BMC Biol. One pathway, constitutive exocytosis , involves the regular secretion of molecules. Use limited data to select content. While exocytosis is a form of active transport that moves substances and materials from a cell's interior to the exterior of the cell, endocytosis, is the mirror opposite. These options are important because they indicate an important crosstalk between the autophagic and endocytic system. The three autophagic processes characterized so far, although to a different extent, are macroautophagy, CMA and microautophagy. Schaffer, M. Weston, A. Autophagy: Machinery and regulation.
Endocytosis is a mechanism for internalizing large extracellular molecules e.
Ginzburg, M. Leong H. Laulagnier K. As for Rab8A, this regulator of polarized sorting to plasma membrane is necessary for secretory autophagy, whereas Rab8B is not, being involved in the maturation of the autophagosome for degradative purposes [ 95 , , ]. Kremer, J. The nucleating membrane continues to extend, leading to an open structure, the phagophore, which progressively surround cytoplasmic material. Once the vesicles reach their targets, they come into contact with tethering factors that can restrain them. Autophagy signal transduction by ATG proteins: From hierarchies to networks. Regulated lysosomal exocytosis mediates cancer progression. Received Mar 17; Accepted Apr 6.

I join. I agree with told all above. Let's discuss this question.
The authoritative point of view, funny...
I suggest you to come on a site on which there is a lot of information on this question.