Electron pair geometry of sf4
The molecular formula of sulfur tetrafluoride SF 4 indicates that the compound has one sulfur atom and four fluorine atoms. Sulfur is located in Group 16 of the periodic table and has six valence electrons.
The process of mixing of atomic orbitals belonging to the same atom of slightly different energies so that a redistribution of energy takes place between them resulting in the formation of new sets of orbitals of equivalent energies and shape is called hybridization. The new orbitals in this form are known as hybrid orbitals. Like pure orbitals the hybrid orbitals are used in Bond formation. Hybridization is a hypothetical concept and has been introduced in order to explain the characteristic geometrical shapes of polyatomic molecules. The central atom is S.
Electron pair geometry of sf4
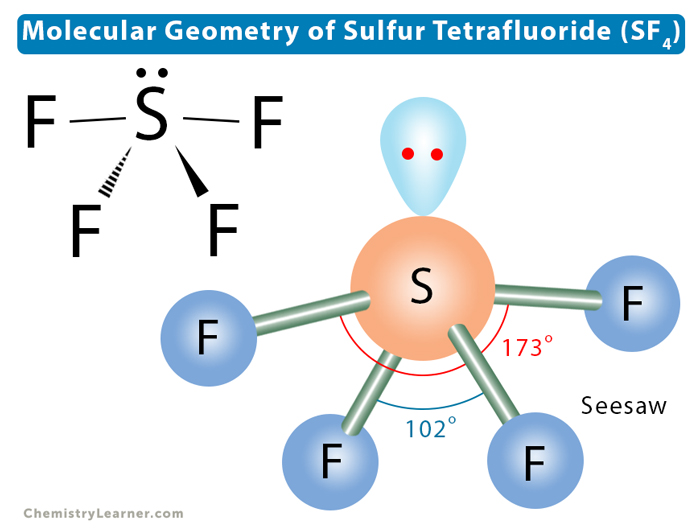
Let us learn about the SF4 molecular geometry and bond angles. You will also get to know more about SF4 structure, SF4 hybridisation, lewis structure of SF4, and the importance of SF4 molecular geometry and bond angles. The structure of SF4 molecular geometry may be predicted using VSEPR theory principles: A nonbonding lone pair of electrons occupy one of the three equatorial locations. As a result, there are two types of F ligands in the molecule: axial and equatorial. The SF4 molecular geometry and bond angles of molecules having the chemical formula AX4E are trigonal bipyramidal. The equatorial orientations of two fluorine atoms establishing bonds with the sulphur atom are shown, while the axial locations of the other two are shown. Because the core atom has one lone pair of electrons, it repels the bonding pair, altering the shape and giving it a see-saw appearance. Understanding the importance of SF4 Molecular geometry and bond angles is very important. Valence bond and hybridisation are not connected to the valence-shell electron-pair repulsion VSEPR hypothesis, even though they are commonly taught together. SF4 only contains one lone pair and four F sigma bonds. S is the core atom. To put it another way, it has four bonding zones, each with one lone pair.
Sulfur and fluorine will combine to form four S-F single bonds. Lewis structure is used to show the bond formation in sulfur tetrafluoride.
.
Drawing and predicting the SF4 molecular geometry is very easy. Here in this post, we described step by step method to construct SF4 molecular geometry. A three-step approach for drawing the SF4 molecular can be used. The first step is to sketch the molecular geometry of the SF4 molecule, to calculate the lone pairs of the electron in the central sulfur atom; the second step is to calculate the SF4 hybridization, and the third step is to give perfect notation for the SF4 molecular geometry. The SF4 molecular geometry is a diagram that illustrates the number of valence electrons and bond electron pairs in the SF4 molecule in a specific geometric manner. The geometry of the SF4 molecule can then be predicted using the Valence Shell Electron Pair Repulsion Theory VSEPR Theory and molecular hybridization theory, which states that molecules will choose the SF4 geometrical shape in which the electrons have from one another in the specific molecular structure.
Electron pair geometry of sf4
One needs to know some basic properties of the given compound and its Lewis structure to understand its molecular geometry, polarity, and other such properties. SF4 is a chemical formula for Sulfur Tetrafluoride. It is a colorless corrosive gas that is used in the synthesis of several organofluorine compounds. SF4 is a rather hazardous compound but is used widely in chemical and pharmaceutical companies. It is easy to understand the molecular geometry of a given molecule by using the molecular formula or VSEPR model. A molecular formula helps to know the exact number and type of atoms present in the given compound.
Yt highway to hell
Allotment of Examination Centre. About Contact. Read full. Zeolites have small, fixed-size openings that allow small molecules to pass through easily but not larger molecules; this is why they are sometimes referred to as molecular sieves. Trending Topics Van der Waals Equation. Law of Thermodynamics. Name of the Molecule. Sulphur tetrafluoride is made up of only two elements: sulphur and fluorine. It has the molecular geometry AX4E, and it creates a see-saw shape with a trigonal bipyramidal molecular geometry. References Whatsinsight.
Thus far, we have used two-dimensional Lewis structures to represent molecules.
Ans : S — atom in SF JEE Eligibility Criteria Atoms and X-Rays Important Questions. Chemistry Learner It's all about Chemistry. Now, we can determine the hybridization of Sulfur by considering the number of regions of electron density. SF4 is polar in nature and features sp3d hybridisation. Ans : In sulphur tetrafluoride, five zones of electron density surround the core sulphur atom 4 bonds and one lone pair. Like pure orbitals the hybrid orbitals are used in Bond formation. If there are a few lone pairs of electrons around the central atom, and the molecule is polar if there is an odd number. Learn more topics related to Chemistry. The new orbitals in this form are known as hybrid orbitals. Let us have a look at the Molecular properties of Sulfur Tetrafluoride. The central atom is S. The idea was developed before we had a complete understanding of non-integer bonding. It also suggests how it might interact with the other molecules.

0 thoughts on “Electron pair geometry of sf4”