Electron dot structure of hno3
HNO 3 Nitric acid lewis stricture is drawn step by step by using valence electrons of each element.
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter. Chemical Properties. Physical Properties. Intensive vs.
Electron dot structure of hno3
Draw the Lewis structure of HCN. Draw a Lewis structure of nitric oxide, NO. Draw the Lewis structure of B e C l 2. Draw the Lewis structure for S F 6. Draw the structure of : Perchloric acid. In the Lewis structure of acetic acid, there are. Draw the Lewis structure of iodine pentafluoride, I F 5. Draw the structure of an amino acid. Draw the structure of phosphinic acid H 3 P O 2. Draw a Lewis structure for nitrogen trichloride, N C l 3. Draw the Lewis structure of nitric acid, HNO3. Which one of the following molecules contains no pi - bond?
Which of the following is a polar moleule?
Several worked examples relevant to this procedure were given in previous posts please see the Sitemap - Table of Contents Lewis Electron Dot Structures. Nitric acid is a strong oxidizing agent and it dissolves practically all metals except gold and platinum and some other precious metals. As such, is an important raw material for the chemical and pharmaceutical industry. It is mainly used for etching and for the production of pure nitrates. Even though nitric acid was known since the 9th century - alchemists used it to separate gold and silver - its mass production started in when a German chemist Wilhelm Ostwald developed an industrial process. Initially it was used for the production of explosives but today its main use is for the production of fertilizers such as ammonium nitrate. Other main applications is for the production of explosives, nylon precursors and substituted organic compounds.
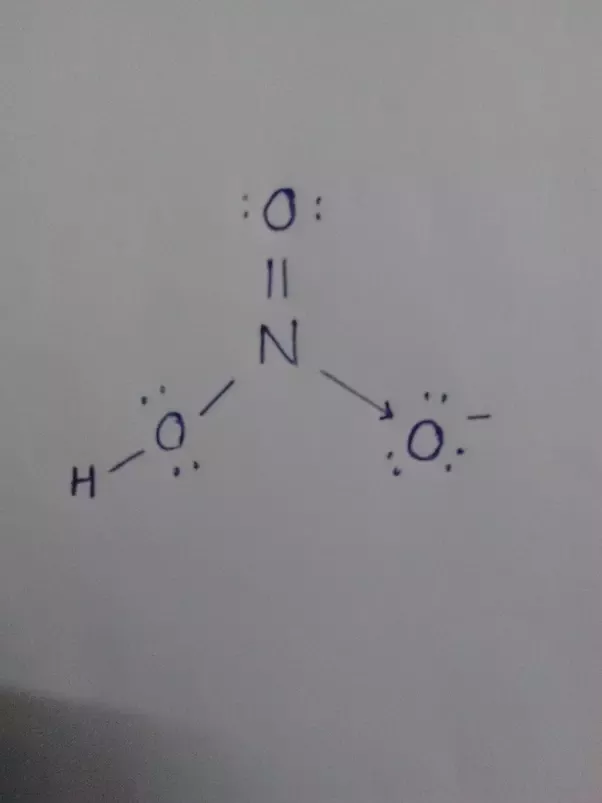
The nitrogen atom is at the center and it is surrounded by 2 oxygen atoms and one O-H bond. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of HNO3. Here, the given molecule is HNO3. In order to draw the lewis structure of HNO3, first of all you have to find the total number of valence electrons present in the HNO3 molecule.
Electron dot structure of hno3
This pattern of adding a hydrogen atom to one of the oxygen atoms is frequently observed in many acids. To determine the total number of valence electrons in the HNO3 molecule and construct the Lewis structure, follow these steps:. Determine the total number of valence electrons. Identify the central atom. In HNO3, the nitrogen atom N is the central atom since it is less electronegative than oxygen. Remember : If hydrogen is present in the molecule, always place the hydrogen atoms on the outside. Place the remaining electrons around the atoms to satisfy the octet rule , except for hydrogen which only needs 2 electrons. Start by placing lone pairs around the oxygen O atoms. Each oxygen atom requires 6 electrons to complete its octet 8 electrons in total, minus the 2 electrons already used for the single bond.
Top follow promo codes
Noble Gas Compounds. Related Videos. Strong-Field vs Weak-Field Ligands. Boron Family: Borane. Naming Benzene. Production of Hydrogen. After determining the center atom and sketch of HNO 3 , we should start to mark lone pairs on atoms. Writing Ionic Compounds. Previous video. The Electron Configuration: Quantum Numbers. In the Lewis structure of acetic acid, there are.
Nitric acid HNO3 , a highly corrosive acid, is a very important chemical. It is usually a colorless liquid, but the older samples turn pale yellow because it gets decomposed into water and oxides of nitrogen.
Video Solution. Total electron pairs are determined by dividing the number total valence electrons by two. Therefore, there should be another bond between nitrogen and that oxygen atom. Naming Coordination Compounds. Initially it was used for the production of explosives but today its main use is for the production of fertilizers such as ammonium nitrate. Periodic Trend: Electronegativity. Dimensional Analysis. Draw the Lewis structure of B e C l 2. The Electron Configurations: Exceptions. Galvanic Cell. Molecular Formula. Chemical Kinetics 2h 43m. Aqueous Equilibrium 4h 42m. Benzene Reactions. Electromagnetic Spectrum.

Between us speaking, I would try to solve this problem itself.