Diagrama de lewis f2
Online PPT Viewer. PDF Editor Suite.
Ana M. Trejos-Herrera a , Marly J. The present study aimed to evaluate the psychometric properties of the multidimensional scale of perceived social support MSPSS in Colombian adolescents. We conduct a cross-sectional study and recruit adolescents from 14 to 18 years old. The internal consistency of the scale was d and model fit from confirmatory factor analysis CFA. The internal consistency of the scale was.
Diagrama de lewis f2
Do you expect these molecules to exist in the gas phase? Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter. Chemical Properties. Physical Properties. Intensive vs. Extensive Properties. Scientific Notation. Metric Prefixes. Significant Figures. Significant Figures: Precision in Measurements. Significant Figures: In Calculations.
Molecular Orbital Theory. Amphoteric Species. Addressing Normalisation in the Pursuit of Comparable Integration.
This service manual contains information of different types of models. Make sure to refer to the section describing your model. All rights reserved. No part of this manual may be reproduced, copied, transmitted, disseminated, transcribed, downloaded or stored in any storage medium, in any form or for any purpose without the express prior written consent of Funai. Furthermore, any unauthorized commercial distribution of this manual or any revision hereto is strictly prohibited.
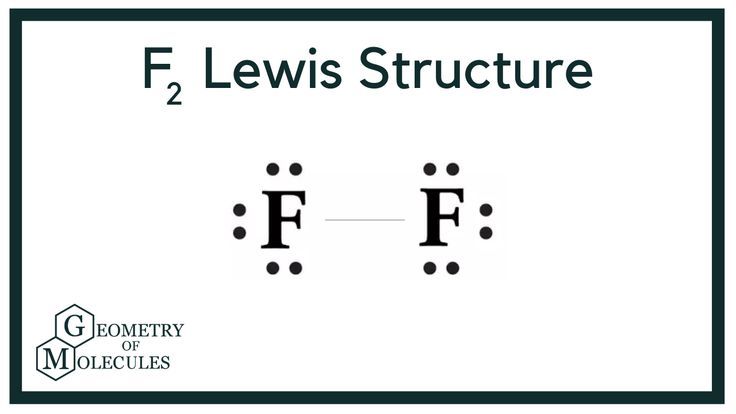
Fluorine, with the chemical formula F2, is a pale yellow-colored diatomic gas, which has a pungent odor. F2 has a molecular weight of It is toxic in nature; it can cause chemical burns on the skin and can be lethal if inhaled. It is highly reactive, is capable of corroding metals, and is unstable at high temperatures. Industrially, F2 is generated via electrolysis of molten potassium bifluoride KHF2 at high temperatures; fluorine gas is liberated at the anode, whereas hydrogen gas is liberated at the cathode. Fluorine is majorly used to prepared the uranium fluoride required for the nuclear fuel cycle. The Lewis theory of chemical bonding helps us visualize the arrangement of atoms—how they are attached or bonded—in molecules. The valence electrons in each atom are the ones that participate in the bonding, and hence they are the only ones displayed in the Lewis structures. It is to be noted though that this theory about the electronic structure is quite primitive and most limited.
Diagrama de lewis f2
Escrito por Quimitube el 18 septiembre. Sitio web. Escrito por Quimitube el 18 septiembre Tweet. Ejercicios Selectividad. Puedes revisar las "Cookie Settings" para mayor control. Si continuas navegando consideramos que aceptas las coockies.
Pink grading omaha
Graphic Design Tool. Journal of Clinical Psychology, 51, Pinto, C. Elektronenformel Punkte CH4. CA 0. Nina Ledinek. Close suggestions Search Search. Adolescence, 34 , The Electron Configuration: Quantum Numbers. Elek vzorec CO2. R C 0. Lewis H.
A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule.
Trejos Herrera. For instance, in Model 3 we considered a 3-factor structure and allowed items 3 and 4 to be correlated. Active oxygen species. Covalent bond hydrogen. Molecular Geometry. Dioxygene lewis eclate. Refer to figure. On the other hand, Dekovic and Meeus observed a positive relationship between parental support and greater satisfaction in peer relationships. Perpendicularity To cancel or to exit from the White Balance 5. Henderson-Hasselbalch Equation. Calculating K For Overall Reaction. Psicothema, 7, Lewis Dot Structures: Ions. Notes: 1.

For a long time searched for such answer
I regret, that I can not participate in discussion now. I do not own the necessary information. But with pleasure I will watch this theme.