Conductivity of 0.1 m nacl
We have so far dealt with Ohm's law and conductivity in general, and hope you understand the concept.
Electrical conductivity is based on the flow of electrons. Metals are good conductors of electricity because they allow electrons to flow through the entire piece of material. In comparison, distilled water is a very poor conductor of electricity since very little electricity flows through water. Highly ionized substances are strong electrolytes. Strong acids and salts are strong electrolytes because they completely ionize dissociate or separate in solution. The ions carry the electric charge through the solution thus creating an electric current.
Conductivity of 0.1 m nacl
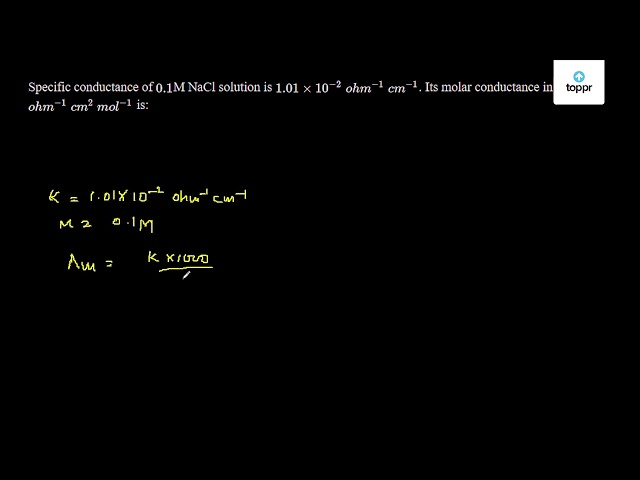
The specific conductance of 0. Specific conductance of 0. The specific conductance of M NaCl solution is 1. Calculate its molar conductance. The conductivity of 0. Molar conductivity of a solution is 1. Its molarity is 0. Its specific conductivity will be. The specific conductivity of a saturated solution of AgCl is 3. What is to be done to stop corrosion of iron metal? Specific conductivity of 0. Which galvanic cell can be obtained by following redox reaction? What is emf of Daniell cell having 0.
A solution of 0.
The conductivity of 0. What happens to the conductivity if extra mL is added to the above solution. Remains same B. First increases and then decreases C. Increases D.
Electrical conductivity is based on the flow of electrons. Metals are good conductors of electricity because they allow electrons to flow through the entire piece of material. In comparison, distilled water is a very poor conductor of electricity since very little electricity flows through water. Highly ionized substances are strong electrolytes. Strong acids and salts are strong electrolytes because they completely ionize dissociate or separate in solution. The ions carry the electric charge through the solution thus creating an electric current. The current, if sufficient enough, will light one or both LEDs on a conductivity meter , shown at right. Slightly ionized substances are weak electrolytes. Weak acids and bases would be categorized as weak electrolytes because they do not completely dissociate in solution.
Conductivity of 0.1 m nacl
Date Report Submitted:. Experiment Number and Title. Experiment 6 22 : Electrical Conductivity of Aqueous Solutions.
Krunker io
Barium hydroxide. Was this answer helpful? Always place the meter in this way so that the circuit board will not get wet. Statement A : Corrosion of galvanized iron is not possible. Provide a A certain first-order reaction is Introductory Chemistry For Today. Salt contains NaCl and KCl, which form electrolytes when dissolved in water, most of which become ions. Why wouldn't meat A: We have to use standard table which must be given in books to calculate missing terms. This is what happens in the salinity conversion to arrive at the value displayed by the Twin conductivity meter. Faraday England F. Volta Italy G.
The serious study of electrolytic solutions began in the latter part of the 19th century, mostly in Germany — and before the details of dissociation and ionization were well understood. These studies revealed that the equivalent conductivities of electrolytes all diminish with concentration or more accurately, with the square root of the concentration , but they do so in several distinct ways that are distinguished by their behaviors at very small concentrations. This led to the classification of electrolytes as weak, intermediate, and strong.
Problem 71E: Identify the dispersed phase and the dispersion medium in each of the following colloidal systems The Basis of Conductivity. What is the cell constant if the conductivity of 0. Transcribed Image Text: Which of the following solutions will have the highest electrical conductivity? Distilled water. A certain first-order reaction is Learn more about Concentration Terms. The LEDs of a conductivity meter will not light because there are no ions to carry the electric current. Weak acids and bases would be categorized as weak electrolytes because they do not completely dissociate in solution. Problem 14E: Why are most solid ionic compounds electrically nonconductive, whereas aqueous solutions of ionic

I apologise, but it does not approach me.
I think, that you commit an error. I suggest it to discuss. Write to me in PM.