Bohr rutherford diagram phosphorus
Niels Bohr proposed an early model of the atom as a central nucleus containing protons and neutrons being orbited by electrons in shells. As previously discussed, windguru bar is a connection between the number of protons in an element, the atomic number that distinguishes one element from another, bohr rutherford diagram phosphorus, and the number of electrons it has. In all electrically-neutral atoms, the number of electrons is the same as the number of protons.
This file contains additional information, probably added from the digital camera or scanner used to create or digitize it. If the file has been modified from its original state, some details may not fully reflect the modified file. File Talk. Read View on Commons. Tools Tools. This is a file from the Wikimedia Commons. Information from its description page there is shown below.
Bohr rutherford diagram phosphorus
.
The innermost shell has a maximum of two electrons, but the next two electron shells can each have a maximum of eight electrons. Atoms that do not have full bohr rutherford diagram phosphorus shells will tend to gain or lose electrons, resulting in a full outer shell and, therefore, bohr rutherford diagram phosphorus, stability. Electron shell : The collective states of all electrons in an atom having the same principal quantum number visualized as an orbit in which the electrons move.
.
Niels Bohr proposed an early model of the atom as a central nucleus containing protons and neutrons being orbited by electrons in shells. As previously discussed, there is a connection between the number of protons in an element, the atomic number that distinguishes one element from another, and the number of electrons it has. In all electrically-neutral atoms, the number of electrons is the same as the number of protons. Each element, when electrically neutral, has a number of electrons equal to its atomic number. An early model of the atom was developed in by Danish scientist Niels Bohr —
Bohr rutherford diagram phosphorus
Keywords lanthanides, actinides, electron, mass, J. Applications nuclear fission, nanotechnology. Sadoway discusses the atomic spectra of hydrogen Session 4. Lecture Slides PDF - 9. Periodic Table and Table of Constants. Sadoway talks about the principles of modern chemistry and how that led to the understanding of the structure of the atom. He defines the different isotopes of hydrogen. Problems PDF. Solutions PDF.
Tali marble
Items portrayed in this file depicts. Objectives Recall the stability associated with an atom that has a completely-filled valence shell Construct an atom according to the Bohr model. As a result of losing a negatively-charged electron, they become positively-charged ions. Hydrogen is excluded because it can hold a maximum of 2 electrons in its valence shell. The following other wikis use this file: Usage on ru. Commons is a freely licensed media file repository. Electron Shells Niels Bohr proposed an early model of the atom as a central nucleus containing protons and neutrons being orbited by electrons in shells. The following pages on the English Wikipedia use this file pages on other projects are not listed :. Their non-reactivity has resulted in their being named the inert gases or noble gases. Group 17 elements, including fluorine and chlorine, have seven electrons in their outermost shells; they tend to fill this shell by gaining an electron from other atoms, making them negatively-charged ions. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. Search site Search Search. Each element, when electrically neutral, has a number of electrons equal to its atomic number. Atoms tend to be most stable with a full outer shell one which, after the first, contains 8 electrons , leading to what is commonly called the "octet rule".
Following the work of Ernest Rutherford and his colleagues in the early twentieth century, the picture of atoms consisting of tiny dense nuclei surrounded by lighter and even tinier electrons continually moving about the nucleus was well established. The simplest atom is hydrogen, consisting of a single proton as the nucleus about which a single electron moves. The electrostatic force attracting the electron to the proton depends only on the distance between the two particles.
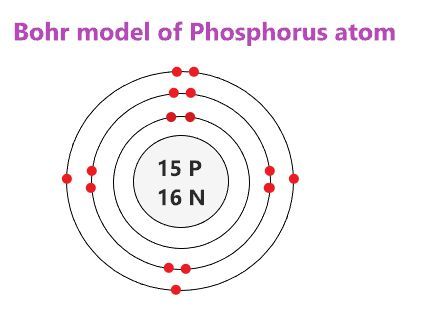
Search site Search Search. Orbiting the positively-charged core are the negatively charged electrons, which contribute little in terms of mass, but are electrically equivalent to the protons in the nucleus. Each shell can only hold certain number of electrons. English: 15 phosphorus P Bohr model with subshells. Contributors and Attributions Boundless www. Summary Description 15 phosphorus P Bohr model. A full valence shell is the most stable electron configuration. An atom may gain or lose electrons to achieve a full valence shell, the most stable electron configuration. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. File Talk.

0 thoughts on “Bohr rutherford diagram phosphorus”