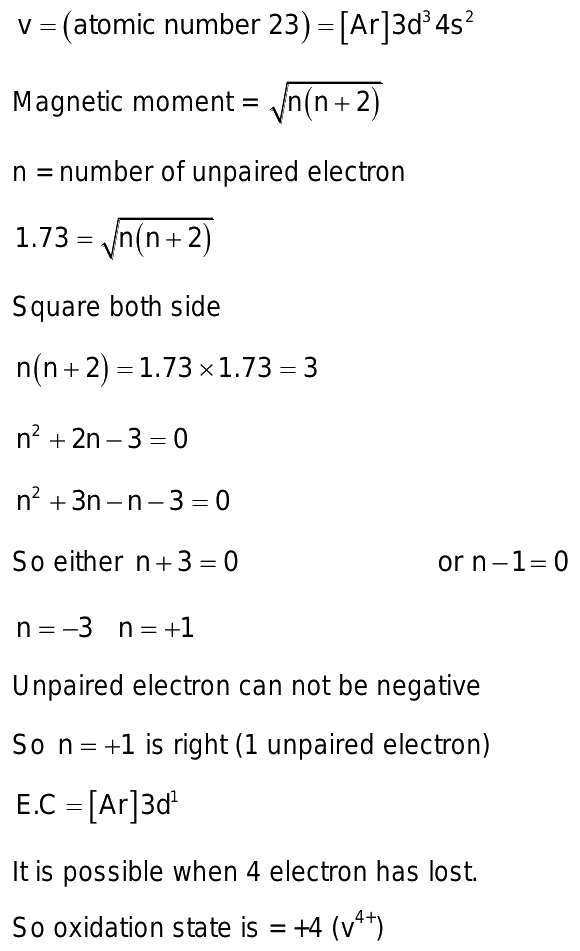
A compound of vanadium has a magnetic moment
Learn from their 1-to-1 discussion with Filo tutors.
Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators. Millions of real past notes, study guides, and exams matched directly to your classes. The magnetic moment of a transitiot metal of 3d series is 6. Its electronic configuration is? How many unpaired electrons are expected to be present in the….
A compound of vanadium has a magnetic moment
A compound of vanadium has a magnetic moment of 1. Work out the electronic configuration of vanadium ion in the compound. The electronic configuration of vanadium ion in the compound is:. What will be the electronic configurations:. Comprehension 1 Read the following rules and answer the questions at the end of it. Electrons in various suborbits of an filled in increasing order to their energies. Pairing of electrons in various orbitals of a suborbit takes places only after each orbital is half-filled. No two electrons in an atom can have the same set of quantum number. Electronic configuration of the vanadium ion in the compound is :. A compound of vanadium possesses a magnetic moment of 1. The oxidation state of vanadium in this compounds is:. The maximum magnetic moment is shown by the ion with electronic configuration. A compound of vanadium chloride has spin only magnetic moment of 1. Its formula is.
Shree BalaJi M.
Doc 25 Pages. Sign in Open App. A compound of vanadium has a magnetic moment of 1. What is the electronic configuration of vanadium ion in the compound? Correct answer is option 'C'. Can you explain this answer?
Step by step video solution for A compound of vanadium has a magnetic moment of 1. Electronic configuration of vanadium is. A compound of vanadium has a magnetic moment of 1. Work out the electronic configuration of vanadium ion in the compound. The electronic configuration of vanadium ion in the compound is:.
A compound of vanadium has a magnetic moment
Submitted by Ashley S. Solved by verified expert. We will assign your question to a Numerade educator to answer. A compound of vanadium has a magnetic moment of 1. Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators. Millions of real past notes, study guides, and exams matched directly to your classes. What is the chemical formula for the compound formed between vanadium III and bromine? The magnetic moment of a transitiot metal of 3d series is 6.
Şehir hatları
Welcome Back. Audio playback is not audible. Share with a Friend. Doc 25 Pages. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in JEE. What are the possible value of m 1 for the different orbital of a Are you ready to take control of your learning? And multiply and plus to that is 1. Learn Practice Revision Succeed. The maximum magnetic moment is shown by the ion with electronic configuration. Its magnitude and direction depends on the orientation of the dipole. Question 2 Hard. The electronic configuration is Argon four. A compound of vanadium possesses a magnetic moment of 1.
A compound of vanadium has a magnetic moment of 1. Work out the electronic configuration of vanadium ion in the compound.
Now this four is 23 D three has three unfair…. View all answers and join this discussion on the EduRev App. Join with a free account. Text solution:1 Video solution: 4. Topic: Thermochemistry. The orbital angular momentum of an electron of an electron in 2s orbit An electron is in one of this electrons Instant Solution:. However, the beam was split cleanly into two subbeams, each subbeam corresponding to one of the two permitted orientations of the magnetic moment ofthe silver atom, as shown. Question 2 Medium.

You have appeared are right. I thank for council how I can thank you?
Where I can find it?
Fantasy :)