2cu o2
Define a chemical equation. Classify the following into physical and chemical changes. Freezing of water.
Last updated on Feb 23, Get Started. SSC Exams. Banking Exams. Teaching Exams. Civil Services Exam. Railways Exams.
2cu o2
Last updated on Feb 23, Get Started. SSC Exams. Banking Exams. Teaching Exams. Civil Services Exam. Railways Exams. Engineering Recruitment Exams. Defence Exams. State Govt. Police Exams. Insurance Exams. Nursing Exams. Other Govt.
IB Security Assistant. Telangana Police Constable. Puneet Chaudhary.
.
Wiki User. Exothermic, combustion. Cu2 should be '2Cu' two uncombined atoms of copper. O2- should be 'O2; 2 combined atoms of oxygen. Thus, copper is oxidized; loses electrons. Tags Chemistry Elements and Compounds Subjects. Log in. Study now See answers 2. Best Answer. I dont no help.
2cu o2
In association with Nuffield Foundation. Copper foil is folded into the shape of an envelope before being heated in a Bunsen burner. On cooling, the foil can be opened and it can be seen that where there was no contact with oxygen the copper remained unreacted. In this experiment, students fold a piece of copper foil into the shape of an envelope, before heating it using a Bunsen burner. When the foil has cooled, students can open the envelope and discover that where there was no contact with oxygen the copper remained unreacted. Warn students that there can be sharp corners on the copper. The copper stays hot for some time and there is a risk of burns.
Uber rides
Mazagon Dock Shipbuilders Non Executive. ISRO Technician. Gujarat Metro Maintainer. MP Vyapam Group 2. TS TET. Haryana B. India Post. DHS Assam Grade 4. Telangana High Court Process Server. Maharashtra Nagar Parishad Accountant. Defence Exams. RRB Staff Nurse. Puducherry Home Guard. CG Vyapam Assistant Teacher.
The oxidation-reduction or in short redox reaction is one of the most common types of chemical reactions happening in and around us. For example, rusting of metals, photosynthesis, digestion of food, and combustion of fuels are redox reactions. The oxidation-reduction reactions happen in a pair because one thing loses electrons and is oxidized; the other thing gains the electrons and is reduced.
Chhattisgarh Junior Engineer. Agricultural Field Officer - Scale I. RBI Security Guard. Rajasthan Housing Board Junior Accountant. BPSC Lecturer. Nainital Bank Clerk. WB TET. Marketing Officer - Scale I. Odisha Police ASI. RRB Paramedical Staff. Punjab Pre-Primary Teacher. Chandigarh Police ASI. Krushi Vibhag Maharashtra Senior Clerk. Delhi Police Driver. Indian Navy Tradesman.

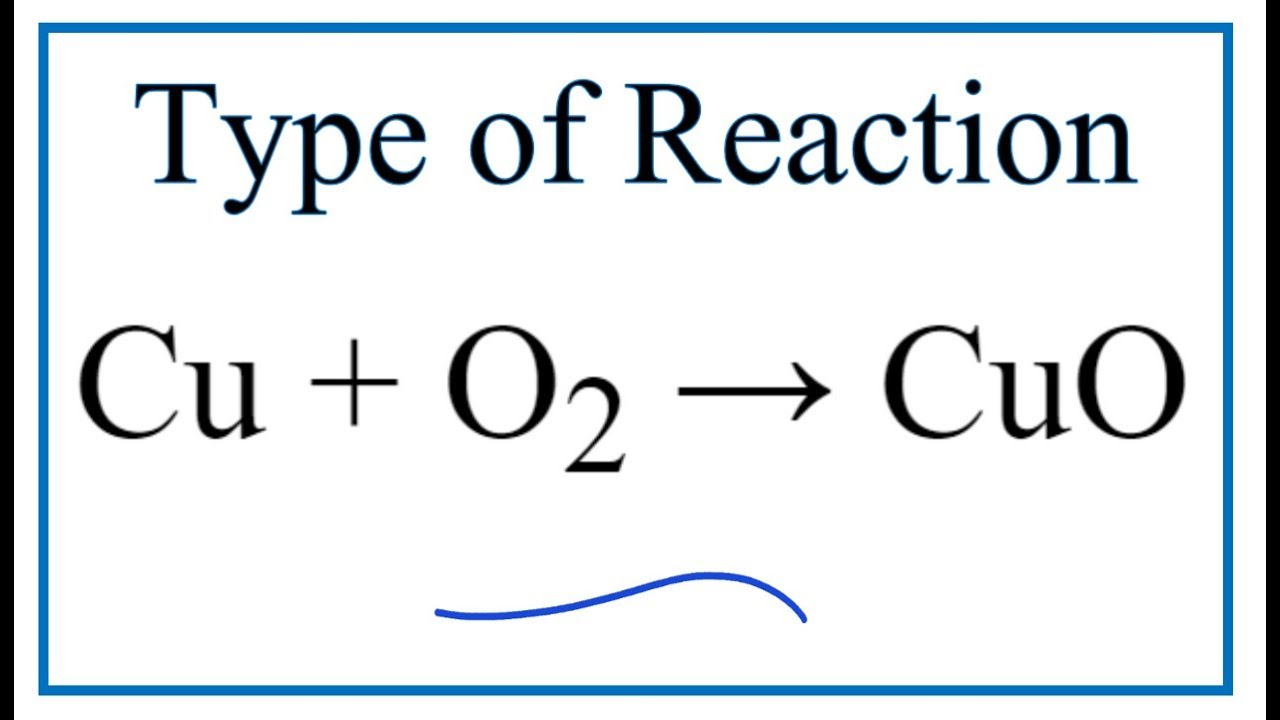
Excuse, it is cleared