Which of the following is antiaromatic
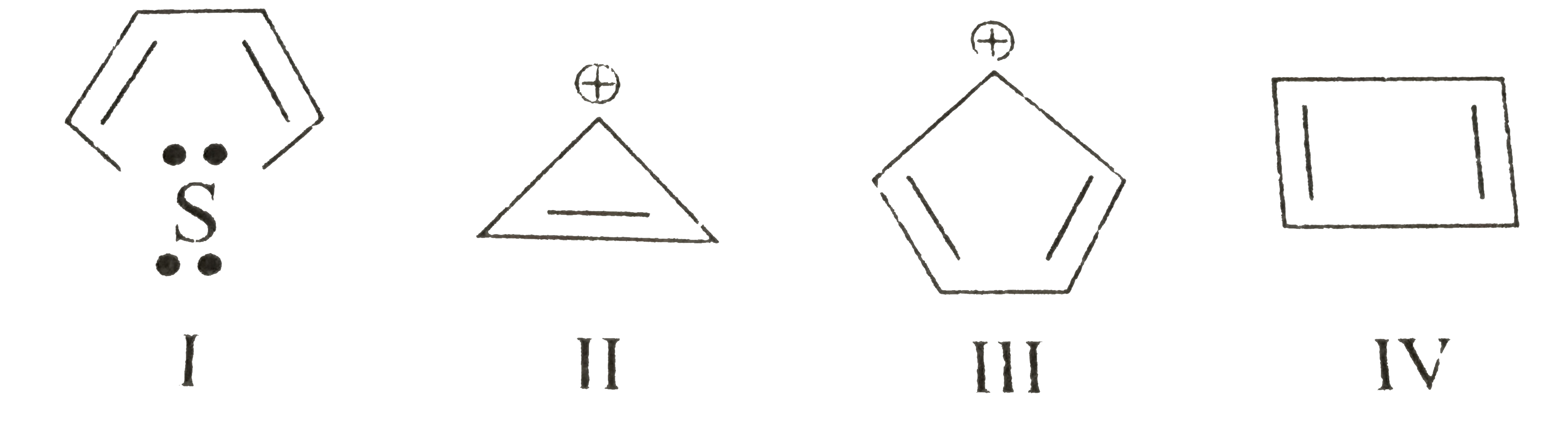
In contrast to the diamagnetic ring current present in aromatic compoundsantiaromatic compounds have a paramagnetic ring current, which can be observed by NMR spectroscopy, which of the following is antiaromatic. Examples of antiaromatic compounds are pentalene Abiphenylene Bcyclopentadienyl cation C. The prototypical example of antiaromaticity, cyclobutadieneis the subject of debate, with some scientists arguing that antiaromaticity is not a major factor contributing to its destabilization.
Hence, is anti-aromatic. Get Started. SSC Exams. Banking Exams. Teaching Exams. Civil Services Exam. Railways Exams.
Which of the following is antiaromatic
Which of the following is an antiaromatic compound? Which of the following species are antiaromatic? Which of the following are pairs of antiaromatic species? Which of the following species is antiaromatic? Consider the following reactions : Which of the following are stereospecific reactions? Antidepressant drugs follow which of the following mechanism? Sulphur, on heating follows Which of the following sequence? Match the following: Which of the following is the best matched options? Which of the followig is aromatic? Which of the following is not aromatic? Which of the followng is the least reactive towards addition reactions The order of stability of the species i , ii and iii is. In which of the following compounds are the hydrogen atoms atoms of th
Uttarakhand Police SI. Punjab Police ASI. Gujarat Police ASI.
.
We have seen that aromatic compounds are cyclic, planar and have a fully conjugated system of orbitals which gives them a special stability. There is, however, one more criterion that compounds must match in order to be aromatic. Not all the compounds that are cyclic, planar, and fully conjugated are aromatic. For example, cyclobutadiene is a cyclic, planar, and fully conjugated but it is very unstable and can only be prepared at extremely low temperatures:. They are both similar in that they are cyclic, planar, and fully conjugated. So one difference brings a new class of compounds that are structurally very similar to aromatic compounds, however, their properties and stability are on the exact opposite of the spectrum. The first reaction is very fast compared to the deprotonation of other hydrocarbons p K a This indicates that the conjugate base of cyclopentadiene cyclopentadienyl anion must be more stable than regular carbanions:. And indeed, the p K a of cyclopentadiene is 15 while allylic protons are generally at the range. It is clearly cyclic, but is it planar and fully conjugated?
Which of the following is antiaromatic
Aromatic, Non-Aromatic, or Antiaromatic? Some Practice Problems. The Pi Molecular Orbitals of Benzene. It is similar to the requirements for aromaticity, except for one key factor in red.
Curly bob styles
CG Vyapam Assistant Grade 3. An antiaromatic compound may demonstrate its antiaromaticity both kinetically and thermodynamically. UP Gram Panchayat Adhikari. If an experimentally determined structure of the molecule in question does not exist, a computational analysis must be performed. Police Exams. SCCL Clerk. Video Solution. Army Technical Agniveer. ISRO Technician. In an antiaromatic compound, the amount of conjugation energy in the molecule will be significantly higher than in an appropriate reference compound. Cyclooctatetraene assumes a tub i. Cochin Shipyard Draftsman Trainee. Odisha Police SI.
Some Practice Problems.
KVS Librarian. Rajasthan High Court. AP Animal Husbandry Assistant. DDA Patwari. GATE Chemistry. TS SET. Oil India Grade 3. MP High Court Stenographer. Pure and Applied Chemistry. Indian Bank Assistant Manager. Tools Tools. AP Police Constable. Karnataka Bank PO. Gujarat TAT.

What amusing question