What is the specific heat of a substance
If a swimming pool and wading pool, both full of water at the same temperature, were subjected to the same input of heat energy, the wading pool would certainly rise in temperature more quickly than the swimming pool. The heat capacity of an object depends both on its mass and its chemical composition.
Heat capacity is an extensive property, so it scales with the size of the system. For example, if it takes 1, J to heat a block of iron, it would take 2, J to heat a second block of iron with twice the mass as the first. The heat capacity of most systems is not a constant. Rather, it depends on the state variables of the thermodynamic system under study. In particular, it is dependent on temperature itself, as well as on the pressure and the volume of the system, and the ways in which pressures and volumes have been allowed to change while the system has passed from one temperature to another. The temperature dependence is why the definition a calorie is formally the energy needed to heat 1 g of water from
What is the specific heat of a substance
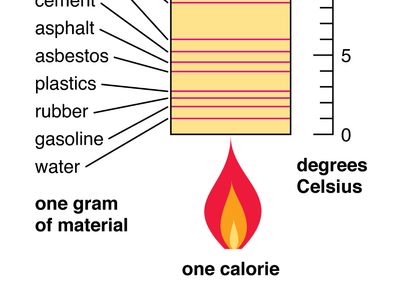
When summer hits, you might end up going to the beach to cool down. While the ocean waves may feel cool, the sand, unfortunately, is red-hot. If you aren't wearing shoes, it's possible to actually burn your feet! Explore our app and discover over 50 million learning materials for free. But how can the water be so cold, but the sand be so hot? Well, that's because of their specific heat. Substances like sand have a low specific heat, so they heat up quickly. However, substances like liquid water have high specific heats, so they are much harder to heat up. In this article, we will be learning all about specific heat: what it is, what it means, and how to calculate it. Specific heat or specific heat capacity C p is the heat capacity divided by the mass of the sample. Basically, specific heat tells us how easily a substance's temperature can be raised. The larger the specific heat, the more energy it takes to heat it. When you are referencing specific heat tables, please pay attention to units!
Starting from the fundamental thermodynamic relation one can show. We also use third-party cookies that help us analyze and understand how you use this website.
In thermodynamics , the specific heat capacity symbol c of a substance is the heat capacity of a sample of the substance divided by the mass of the sample, also sometimes referred to as massic heat capacity or as the specific heat. Specific heat capacity often varies with temperature, and is different for each state of matter. The specific heat capacity of a substance, especially a gas, may be significantly higher when it is allowed to expand as it is heated specific heat capacity at constant pressure than when it is heated in a closed vessel that prevents expansion specific heat capacity at constant volume. Specific heat capacity is also related to other intensive measures of heat capacity with other denominators. One of the first scientists to use the concept was Joseph Black , an 18th-century medical doctor and professor of medicine at Glasgow University. He measured the specific heat capacities of many substances, using the term capacity for heat.
In equation form, this can be represented as the following:. That is if a constant has units, the variables must fit together in an equation that results in the same units. So C equals something with energy in the numerator and temperature in the denominator. Now, you need to use some common sense here, as we are adding heat, not work, and adding heat changes the temperature, it does not make the temperature. In words, heat capacity is the substance's ability to resist change in temperature upon exposure to a heat source. A substance with a small heat capacity cannot hold a lot of heat energy and so warms up quickly. On the other hand, a substance with a high heat capacity can absorb much more heat without its temperature drastically increasing.
What is the specific heat of a substance
Heat capacity is an extensive property, so it scales with the size of the system. For example, if it takes 1, J to heat a block of iron, it would take 2, J to heat a second block of iron with twice the mass as the first. The heat capacity of most systems is not a constant. Rather, it depends on the state variables of the thermodynamic system under study. In particular, it is dependent on temperature itself, as well as on the pressure and the volume of the system, and the ways in which pressures and volumes have been allowed to change while the system has passed from one temperature to another. The temperature dependence is why the definition a calorie is formally the energy needed to heat 1 g of water from
Summary of act 2 in julius caesar
System properties Note: Conjugate variables in italics Property diagrams Intensive and extensive properties. Solving Problems with Calorimetry Calorimetry is used to measure the amount of heat produced or consumed in a chemical reaction. Classical Statistical Chemical Quantum thermodynamics. When summer hits, you might end up going to the beach to cool down. Heat capacity is an extensive property, so it scales with the size of the system. The calibration is accomplished using a reaction with a known q, such as a measured quantity of benzoic acid ignited by a spark from a nickel fuse wire that is weighed before and after the reaction. Measuring the heat capacity at constant volume can be prohibitively difficult for liquids and solids. Julius Robert Mayer : Julius Robert von Mayer November 25, — March 20, , a German physician and physicist, was one of the founders of thermodynamics. A calorimeter is used to measure the heat generated or absorbed by a physical change or chemical reaction. The thermometer measures the change in heat of the water, which is used to then calculate the specific heat of the substance. If you aren't wearing shoes, it's possible to actually burn your feet! Specific Heat for an Ideal Gas at Constant Pressure and Volume An ideal gas has different specific heat capacities under constant volume or constant pressure conditions.
Specific heat describes the amount of thermal energy required to raise the temperature of one unit mass of a substance by one degree Celsius or Kelvin. It plays a crucial role in understanding how different materials respond to changes in temperature and their ability to store or release thermal energy. The specific heat formula calculates the amount of heat transferred into and out of a system.
A g sample of a silver metal absorbs J of heat, which causes the temperature to rise by 4. Fundamentals of statistical and thermal physics. First, we will define heat capacity and specific heat. These calorimeters are created to withstand high-pressure reactions, hence why it is called a "bomb". As you can see, water has a different specific heat when it is a solid, liquid, and gas. This method is used primarily in academic teaching as it describes the theory of calorimetry. Notice that water has a very high specific heat compared to most other substances. In chemistry, heat amounts were often measured in calories. The classical rule, recognized by Clausius and by Kelvin, is that the pressure exerted by the calorimetric material is fully and rapidly determined solely by its temperature and volume; this rule is for changes that do not involve phase change, such as melting of ice. As another example, many animals rely on freshwater. Cool water from the lake is pumped into the plant, while warmer water is pumped out of the plant and back into the lake.

Good business!
I am sorry, it not absolutely that is necessary for me. There are other variants?