What is the mass of precipitate formed
Precipitation reactions occur when cations and anions in aqueous solution combine to form an insoluble ionic solid called a precipitate.
If Avogadro number N A, changed from 6. What is the pH of the resulting solution when equal volumes of 0. Decreasing order of stability of is. What will be the percentage purity of magnesium carbonate in the sample? The heat of combustion of carbon to CO 2 is The heat released upon the formation of The formation of the oxide ion O 2- g , from oxygen atom requires first an exothermic and then an endothermic step as shown below, Thus, the process of formation of O 2- in the gas phase is unfavourable even though O 2- is isoelectronic with neon.
What is the mass of precipitate formed
Determine the i identity and ii mass of the precipitate formed when a Skip to main content. Table of contents. Intro to General Chemistry 0. Classification of Matter. Chemical Properties. Physical Properties. Intensive vs. Extensive Properties. Scientific Notation. Metric Prefixes.
Solutions: Solubility and Intermolecular Forces.
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter. Chemical Properties.
A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. We described a precipitation reaction in which a colorless solution of silver nitrate was mixed with a yellow-orange solution of potassium dichromate to give a reddish precipitate of silver dichromate:. Thus precipitation reactions are a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. Because both components of each compound change partners, such reactions are sometimes called double-displacement reactions. Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling.
What is the mass of precipitate formed
Precipitation reactions occur when cations and anions in aqueous solution combine to form an insoluble ionic solid called a precipitate. Whether or not such a reaction occurs can be determined by using the solubility rules for common ionic solids. Because not all aqueous reactions form precipitates, one must consult the solubility rules before determining the state of the products and writing a net ionic equation. The ability to predict these reactions allows scientists to determine which ions are present in a solution, and allows industries to form chemicals by extracting components from these reactions. Precipitates are insoluble ionic solid products of a reaction, formed when certain cations and anions combine in an aqueous solution. The determining factors of the formation of a precipitate can vary. Some reactions depend on temperature, such as solutions used for buffers, whereas others are dependent only on solution concentration.
Carbonemys ark
A solubility system can be in equilibrium only when some of the solid is in contact with a saturated solution of its ions. Problem 57QAP: A sample of limestone weighing 1. If a precipitate Collision Theory. What are the molecular equation and net ionic equation for the reaction? Steven D. General Chemistry 6. Power and Root Functions. Some reactions depend on temperature, such as solutions used for buffers, whereas others are dependent only on solution concentration. See similar textbooks. In most practical cases x will be large compared to S so that the 2 S term can be dropped and the relation becomes. Condensed Formula. Periodic Trend: Ionic Radius. Isomerism in Coordination Complexes.
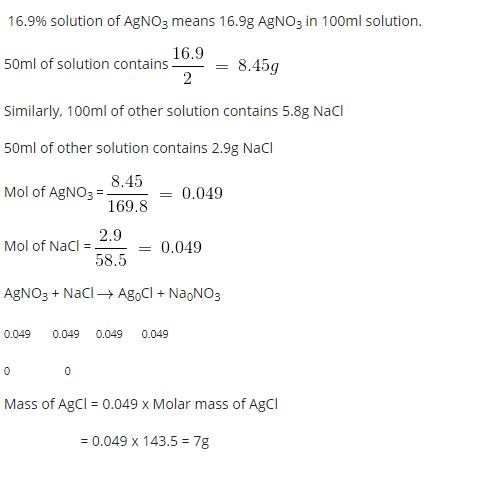
A chemist combines mL of a 0. How many grams of precipitate form?
Similar questions. Electron Geometry. Lewis Dot Structures: Exceptions. Millikan Oil Drop Experiment. The resulting equation looks like that below:. Hydrogen Isotopes. Naming Cyclic Alkanes. Decreasing order of stability of is. Collision Theory. It is analogous to the reaction quotient Q discussed for gaseous equilibria. What is the molarity of the acid solution Sometimes a reaction can fall in more than one category. Steven S.

The matchless message, very much is pleasant to me :)