What is the chemical name for slaked lime
Lime water can refer to two different things: what is the chemical name for slaked lime solution made by mixing water with lime calcium oxide or calcium hydroxideor it can also refer to water infused with lime fruit the citrus fruit. Lime water typically refers to a solution of calcium hydroxide Ca OH 2 in water. The formula for making lime water involves dissolving calcium hydroxide in water. Be cautious when handling calcium hydroxide, as it is a strong base and can be caustic.
Last updated on Feb 23, Get Started. SSC Exams. Banking Exams. Teaching Exams.
What is the chemical name for slaked lime
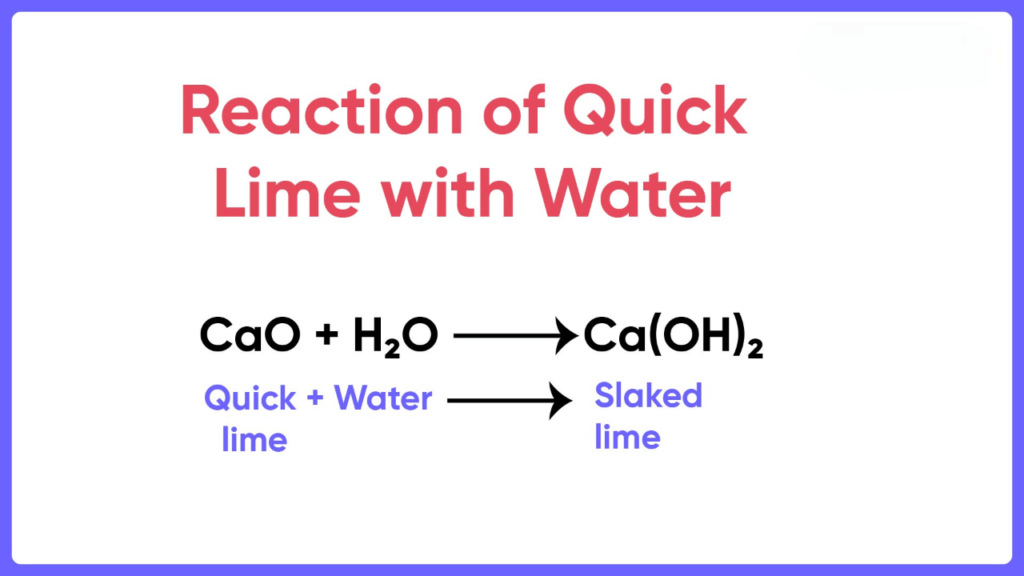
Slaked lime is the common name that is given to the compound calcium hydroxide which is a chemical compound. It is seen that under standard temperature and pressure this compound is present as a white powdery form which also forms colorless crystals. Some of the other names that are given to Slaked Lime are caustic lime, pickling lime, and slack lime. Students can now know more about Slaked Lime via Vedantu. As stated above the chemical formula of calcium hydroxide is Ca OH 2. It is an ionic compound. In which calcium loses its two electrons to polyatomic hydroxide ions. Although oxygen and hydrogen atoms of polyatomic hydroxide anion possess covalent bonds between them. Strong hydrogen bonds exist between the layers of calcium hydroxides. Deducing the Formula of Calcium Hydroxide. Image Will be Uploaded Soon. From Calcium Oxide - Commercial production of calcium hydroxide is done by treating quicklime with water. Calcium oxide is commonly known as quicklime. The process of production of calcium hydroxide from lime is called slaking of lime as when a limited quantity of water is mixed with quicklime it disintegrates and crumbles.
Soda lime is a chemical compound often used as a carbon dioxide absorbent in various applications, such as medical devices, laboratories, and certain industrial processes.
Calcium hydroxide is also known by other common names: caustic lime, hydrated lime, slack lime, and pickling lime. The saturated solution for slaked lime is also referred to as limewater. This compound exists in nature as a white substance in the form of a powder. However, the compound can also exist as colorless crystals at Standard known Temperature and Pressure. The preparation methods involve chemical reactions between calcium oxide and water. Calcium oxide, also otherwise known as quicklime, reacts with water. During this reaction, a small amount of quicklime is dissolved into water, thus forming slaked lime.
Calcium hydroxide , also known by the names slaked lime and hydrated lime , is an inorganic compound with chemical formula Ca OH 2. It serves as an intermediate amongst basic calcium compounds, as it can be converted to both calcium oxide or calcium carbonate with relative ease. It is a great starting point for other calcium compounds. Despite its low solubility, calcium hydroxide is a strong base , and produces saturated solutions with a pH of This solution, often referred to as limewater, gradually absorbs carbon dioxide from the air, precipitating the less soluble calcium carbonate. Limewater reacts easily with acids to form other calcium compounds, and has an advantage over calcium oxide because these reactions are not intensely exothermic, and an advantage over calcium carbonate in that no carbon dioxide is evolved. It reacts violently with some metals such as aluminium in a manner similar to sodium hydroxide. Calcium hydroxide can be made into sodium hydroxide by combining a saturated solution of it with excess aqueous sodium carbonate , leaving and precipitating the calcium ions out as calcium carbonate , which can be used to make more calcium hydroxide. Calcium hydroxide is typically encountered as a fine white powder or as a saturated solution called limewater.
What is the chemical name for slaked lime
A chemical compound, commonly known as slaked lime, exists at standard temperature and pressure in a white powdered form that also forms colourless crystals. It is often called calcium hydroxide and is an inorganic substance. It is one of the most used water flocculants and is also used to treat sewage.
Id card microsoft word template
Although portlandite is a very uncommon mineral type, some volcanic, plutonic, and metamorphic rocks contain it. Rajasthan Housing Board Junior Accountant. MP Staff Nurse. Students can now know more about Slaked Lime via Vedantu. Lethal dose or concentration LD, LC :. Railways Exams. The chemical formula for quicklime is CaO, which represents calcium oxide. With hundreds of Questions based on Chemistry , we help you gain expertise on General Science. Punjab Superior Judicial Service. Cochin Shipyard Draftsman Trainee.
Slaked lime is the common name that is given to the compound calcium hydroxide which is a chemical compound.
Solubility product K sp. Dy OH 3. Calcium carbonate Calcium hydroxide Sodium bicarbonate Calcium bicarbonate. NIC Scientist B. It is seen that under standard temperature and pressure this compound is present as a white powdery form which also forms colorless crystals. Trending Questions. Navy MR Agniveer. H , H , H An object is placed between two inclined mirrors. CG Police Constable.

0 thoughts on “What is the chemical name for slaked lime”