What do you mean by electron gain enthalpy
To define electron gain enthalpysometimes, it is also called Electron affinityalthough there exists a small difference between them. The amount of energy released when an electron is added to an isolated gaseous atom is characterised as an electron gain enthalpy. During the addition of the electron, either the energy can be released or absorbed.
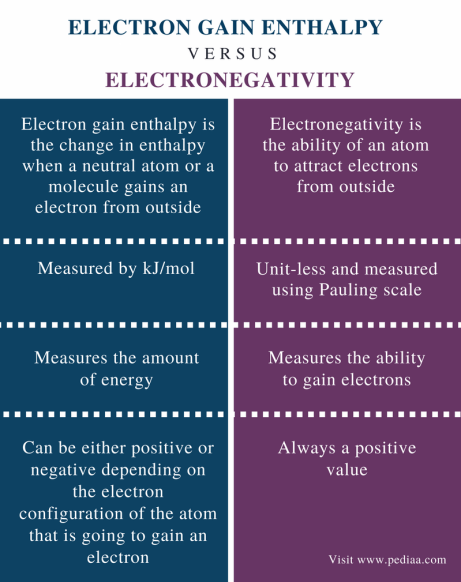
The electron gain enthalpy is an indication of the ability of an atom in gaseous state to release its energy when it gains electrons. It is used in many chemical and physical processes, including photosynthesis and combustion. In general, molecules with higher negative electron gain enthalpies are more reactive and can release energy more easily. The value is negative if the energy is released exothermic and positive if the energy is absorbed endothermic. The electron affinity of an atom is the amount of energy released when an electron is added to a gaseous atom. In chemistry, electron gain enthalpy is the change in enthalpy that accompanies the addition of an electron to an atom gaseous state. In general, the negative EGA decreases as the atomic number of the element increases in a group.
What do you mean by electron gain enthalpy
Electron gain enthalpy is nothing but the energy related to an affinity for the electron of an element. It is a tendency of an element towards electron addition to its outermost shell. The addition of electrons is not so easy as it is an energy-dependent process. The entire process is carried out with an element in its gaseous state. The size of the element has a great impact on the electron gain enthalpy as the nuclear attraction force on the valence shell varies with the size of an element. In this Chemistry article, you will get an idea about the Electron gain enthalpy in detail. Enthalpy is basically energy. Electron gain enthalpy means the energy associated with the addition of an electron into a valence shell of an element in its gaseous form. The electron addition in a valence shell is an energy-driving process and from outside, an electrical moiety is included in another charged region is an energy-consuming process. The nucleus of an atom or element always exerts its attraction force on the valence electrons. The entire process may be energy releasing or energy absorbing that depends on the nature of the element. Electron affinity of halogens is remarkable as they always want to uptake electrons to achieve electronic configuration just like their nearest noble gas. Therefore the electron-gaining process is favourable for them and thus, it follows the exothermic route. If we consider a group, the size of the elements generally enhances moving down the group.
Which element has the most electron gain enthalpy? The amount of energy released when an isolated gaseous atom takes an electron to create a monovalent gaseous anion is known as electron gain enthalpy. Which element has the lowest electron gain enthalpy?
How many of you are aware of what electrons are? But, what is electron gain enthalpy? Well, not anymore! In this chapter, we will look at the concept of electron gain enthalpy and discuss it in greater detail. Electron gain enthalpy of an element is the energy released when a neutral isolated gaseous atom accepts an extra electron to form the gaseous negative Ion i. Greater the amount of energy released in the above process , higher is the electron gain enthalpy of the element. The electron gain enthalpy of an element is a measure of the firmness or strength with which an extra electron is bound to it.
How many of you are aware of what electrons are? But, what is electron gain enthalpy? Well, not anymore! In this chapter, we will look at the concept of electron gain enthalpy and discuss it in greater detail. Electron gain enthalpy of an element is the energy released when a neutral isolated gaseous atom accepts an extra electron to form the gaseous negative Ion i. Greater the amount of energy released in the above process , higher is the electron gain enthalpy of the element. The electron gain enthalpy of an element is a measure of the firmness or strength with which an extra electron is bound to it.
What do you mean by electron gain enthalpy
The reaction can be given as below:. On the basis of the nature of the element, the process of accepting electrons in an atom can either be exothermic or endothermic. In general, energy is released when an electron is added to an atom and the electron gain enthalpy for such elements is negative. The electron gain enthalpy of halogens is highly negative because it needs only one electron to achieve the nearest noble gas configuration.
Chpt quote
Now, let us take two non-metals, Chlorine and Sulphur. The size of the element has a great impact on the electron gain enthalpy as the nuclear attraction force on the valence shell varies with the size of an element. Some external energy is needed to add the electron in their atoms. Since the Magnesium atom is small, the attractive nuclear force will be more on the electrons, whereas the size of the Sodium atom is comparatively larger than that of the Magnesium atom. The electron gain enthalpy results in less negative as we move down a group. Electron Gain Enthalpy of Halogens Electron affinity of halogens is remarkable as they always want to uptake electrons to achieve electronic configuration just like their nearest noble gas. O and F are less negative than the corresponding elements of the third period. Let us look at the difference between electron affinity and electron gain enthalpy in detail. For example, fluorine has a lower negative electron gain enthalpy than chlorine. Table of Content. The unit by which the electron gains enthalpy of an electron is measured in electron volts per atom or kJ per mole. Electronegativity and electron gain enthalpy are the two chemical terms that are used to explain the binding of an electron with an atom.
Electron gain enthalpy is often confused with electron affinity but to make it simpler to understand we can say that it is the energy that is released when a neutral isolated gaseous atom accepts an extra electron and forms a gaseous negative ion, called an anion. It is a measure of the strength with which an extra electron is bound to the element. The greater the amount of energy released in the reaction, the higher is the electron gain enthalpy of the element.
The fluorine atom due to smaller size and high electron-electron repulsion in the valence shell releases less energy than chlorine. After acquiring one electron, the stability of Chlorine will be more. The size of the atom: The smaller the atom, the more tightly bound the electrons are to the nucleus, and thus the more difficult it is for them to be attracted to another electron. See all questions in Enthalpy. Post My Comment. It can be an endothermic or exothermic reaction when you add an electron to the atom. Electronic configuration. The electron affinity of an atom is the amount of energy released when an electron is added to a gaseous atom. With the increase of atomic radius along each period as a row of the periodic table, the electrons that are added move readily to the same outer shell which results in the decrease of the atomic radius due to the varied increase in the nuclear charge. Joanna January 19, at pm. The electron gain enthalpy trends that occur in electron gain enthalpy values within a period are irregular for elements of groups 2, 15 and 18 since they have atoms having symmetrical configuration having half-filled and filled orbitals in the same subshell and thus do not have any urge to take up the extra electrons because their configuration will become either less stable or unsymmetrical. Customize your course in 30 seconds Which class are you in? Hence, the electron gain enthalpy becomes less negative. Electronegativity is the tendency of the atom of an element in a chemical compound to attract a shared pair of electrons towards it in a covalent bond. As a result of its small size, the electron-electron repulsion in the relatively compact to 2p subshell are comparatively large.

Yes... Likely... The easier, the better... All ingenious is simple.
Excuse, I can help nothing. But it is assured, that you will find the correct decision.