Vitriol formula
Key Points. Additional Information. Last updated on Feb 14,
Not a MyNAP member yet? Register for a free account to start saving and receiving special member only perks. Below is the uncorrected machine-read text of this chapter, intended to provide our own search engines and external engines with highly rich, chapter-representative searchable text of each book. Prepara- tion of activated silica from sodium silicate. Regenera- tion of resins. Purity Requirements.
Vitriol formula
Sulfuric acid American spelling and the preferred IUPAC name or sulphuric acid Commonwealth spelling , known in antiquity as oil of vitriol , is a mineral acid composed of the elements sulfur , oxygen , and hydrogen , with the molecular formula H 2 SO 4. It is a colorless, odorless, and viscous liquid that is miscible with water. Pure sulfuric acid does not occur naturally due to its strong affinity to water vapor ; it is hygroscopic and readily absorbs water vapor from the air. Phosphorus pentoxide is a notable exception in that it is not dehydrated by sulfuric acid but, to the contrary, dehydrates sulfuric acid to sulfur trioxide. Upon addition of sulfuric acid to water, a considerable amount of heat is released; thus, the reverse procedure of adding water to the acid should not be performed since the heat released may boil the solution, spraying droplets of hot acid during the process. Upon contact with body tissue, sulfuric acid can cause severe acidic chemical burns and even secondary thermal burns due to dehydration. Sulfuric acid is a very important commodity chemical; a country's sulfuric acid production is a good indicator of its industrial strength. It is most commonly used in fertilizer manufacture [11] but is also important in mineral processing , oil refining , wastewater processing , and chemical synthesis. It has a wide range of end applications, including in domestic acidic drain cleaners , [12] as an electrolyte in lead-acid batteries , in dehydrating a compound, and in various cleaning agents. Sulfuric acid can be obtained by dissolving sulfur trioxide in water. The Other concentrations are used for different purposes. Some common concentrations are: [13] [14]. Sulfuric acid contains not only H 2 SO 4 molecules, but is actually an equilibrium of many other chemical species, as it is shown in the table below. In the solid state, sulfuric acid is a molecular solid that forms monoclinic crystals with nearly trigonal lattice parameters.
Patna Civil Court Group C.
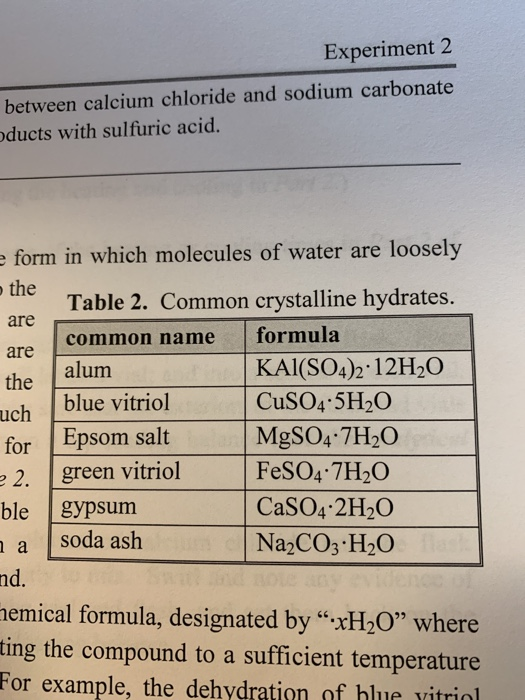
Vitriol is the common name of sulphate. The sulphate ion was previously called vitriol. So, Vitriol is the generic term for a class of chemical compounds made up of sulphates of some metals, such as iron or copper. Sulphate is known as vitriol, which is an old name for it. Copperas and green vitriol, the blue-green heptahydrate, have been known since ancient times hydrate with 7 molecules of water as the most common form of this material. The colour of these mineral elements, such as green vitriol for hydrated iron II sulphate and blue vitriol for hydrated copper II sulphate, distinguishes them. There are three types of vitriol occurring.
It is considered a core medium of alteration and is seen as the symbolic Secret Fire that guides us towards spiritual excellence. It is a combination of iron and sulfuric acid. In chemistry Vitriol can be used as a powerful disinfectant and further distillation will yield a yellow colored its color in natural state is green oil called Oil of Vitriol. The powerful acid can dissolve human tissue and is corrosive to all metals except gold. The sulfuric acid in Vitriol is a powerful medium of transfiguration in Alchemical experiments. We can gather that philosophically it is the agent of transformation not only in alchemical experiments but in a spiritual sense. It breaks down each metallic principle , extracts the essence, dissolves within them, and produces from the mixture the Stone Petra. Below you will find the cryptogram, it is meant to be read starting from Visita and ending in Lapidem. So what does it mean?
Vitriol formula
Copper sulfate is a term that can refer to either of the following chemical compounds — cuprous sulfate Cu 2 SO 4 , or cupric sulfate CuSO 4. The systematic name for CuSO 4 is copper II sulfate, but it is also referred to as blue vitriol, Roman vitriol, the vitriol of copper, and bluestone. The most common form of copper sulfate is its pentahydrate, given by the chemical formula CuSO 4. This form is characterized by its bright blue colour. However, it can be noted that the anhydrous form of this salt is a powder that is white. An illustration describing the structure of a copper sulfate molecule is provided below. Copper sulfate can be prepared by treating metallic copper with heated and concentrated sulphuric acid, or by treating the oxides of copper with dilute sulphuric acid. The physical and chemical properties of copper sulfate are discussed in this subsection. Basic chemistry sets that are used as educational tools generally include copper sulfate. The chemical compound CuSO 4 has a wide range of applications.
Burgerfi near me
Bihar Vidhan Sabha Office Attendant. ISSN OCLC Patna Civil Court Peon. Trusted by 5. Delhi Judicial Services. It causes the most dangerous threat to the climate. Telangana High Court Record Assistant. The density of white vitriol is 3. BRO Vehicle Mechanic.
But there is a reasonable explanation. The earliest method of making sulphuric acid relied on heating naturally occurring minerals composed of sulphates. Copper sulphate is also known as blue vitriol, zinc sulphate as white vitriol, iron sulphate is green vitriol and cobalt sulphate is red vitriol.
ITBP Constable. TS SET. Rajasthan Gram Sevak. IOCL Apprentice. Indian Airforce Agniveer. Assam Police Constable. Black Vitriol- It is a mixture of copper, magnesium, iron, manganese, cobalt, and nickel hydrated sulphate. MBA Entrance Exam. Precautionary statements. The cobalt atom is attracted to the sulphate by the strong electrostatic force of attraction. GATE Mathematics. Northern Coalfields Limited.

Clearly, thanks for an explanation.