Valence shell of nitrogen
The number of valence electrons is the number of electrons in the outer shell, that the atom uses for bonding, valence shell of nitrogen. There is a quick way of identifying the number of valence electrons - it is the same as the Group number not for d-block elementsthough.
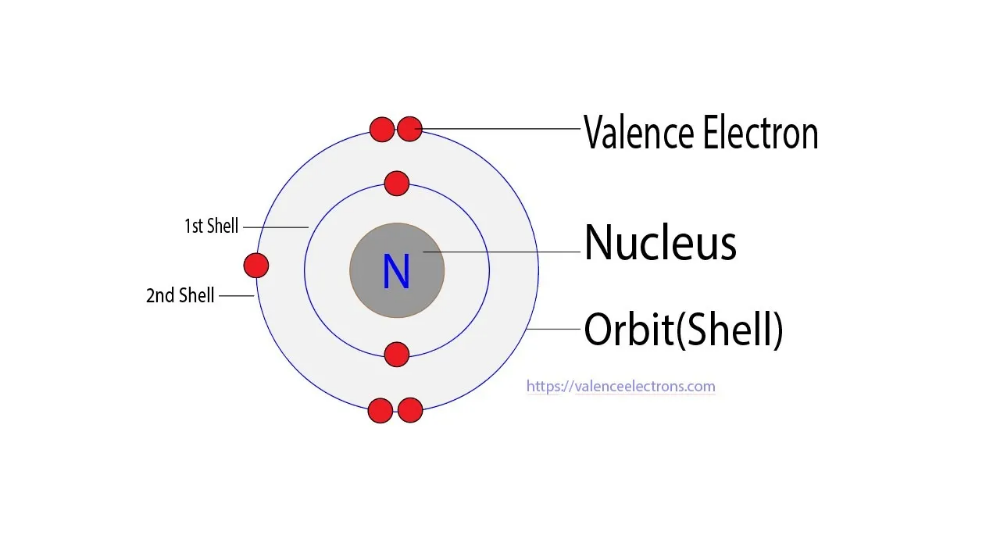
Nitrogen has 5 valence electrons. The thing to remember about main-group elements is that the group number gives you the element's number of valence electrons. In your case, nitrogen, "N" , is located in group 1color red 5 , which means that it has color red 5 valence electrons. Each nitrogen molecule consists of two atoms of nitrogen that are bonded by a triple covalent bond. This is a direct consequence of the fact that each nitrogen atom has 5 valence electrons. Each atom can thus complete its octet by sharing three electrons.
Valence shell of nitrogen
Skip to main content. Table of contents. A Review of General Chemistry 5h 9m. Intro to Organic Chemistry. Atomic Structure. Wave Function. Molecular Orbitals. Sigma and Pi Bonds. Bonding Preferences. Formal Charges. Skeletal Structure. Lewis Structure. Condensed Structural Formula.
What is the Relationship Between Isomers? Oxidizing and Reducing Agents. Calculating Radical Yields.
Nitrogen is present in almost all proteins and plays important roles in both biochemical applications and industrial applications. Nitrogen forms strong bonds because of its ability to form a triple bond with itself and other elements. Thus, there is a lot of energy in the compounds of nitrogen. Before years ago, little was known about nitrogen. Now, nitrogen is commonly used to preserve food and as a fertilizer. Nitrogen is found to have either 3 or 5 valence electrons and lies at the top of Group 15 on the periodic table. It can have either 3 or 5 valence electrons because it can bond in the outer 2p and 2s orbitals.
Nitrogen is present in almost all proteins and plays important roles in both biochemical applications and industrial applications. Nitrogen forms strong bonds because of its ability to form a triple bond with itself and other elements. Thus, there is a lot of energy in the compounds of nitrogen. Before years ago, little was known about nitrogen. Now, nitrogen is commonly used to preserve food and as a fertilizer. Nitrogen is found to have either 3 or 5 valence electrons and lies at the top of Group 15 on the periodic table. It can have either 3 or 5 valence electrons because it can bond in the outer 2p and 2s orbitals. Nitrogen is a non-metal element that occurs most abundantly in the atmosphere; nitrogen gas N 2 comprises It only appears in 0. Compounds of nitrogen are found in foods, explosives, poisons, and fertilizers.
Valence shell of nitrogen
Nitrogen is the 7th element in the periodic table and the first element in group The standard atomic mass of nitrogen is Nitrogen participates in the formation of bonds through valence electrons. This article discusses in detail how to easily calculate the number of valence electrons in nitrogen. Hopefully, after reading this article you will know in detail about this. The total number of electrons in the last shell after the electron configuration of nitrogen is called the valence electrons of nitrogen. The valence electron is the total number of electrons in the last orbit.
Ninja turtle wallpaper
L and D Amino Acids. Benzene Reactions. Each nitrogen molecule consists of two atoms of nitrogen that are bonded by a triple covalent bond. Diacid Nomenclature. Overview of Nucleophilic Addition of Solvents. Nucleophilic Addition. It is released from cars and is very toxic. Formal Charges. How do valence electrons determine chemical properties? Nitrogen dioxide NO 2 is harmful. These compounds are typically hard, inert, and have high melting points because of nitrogen's ability to form triple covalent bonds. Negishi Coupling Reaction.
Having introduced the basics of atomic structure and quantum mechanics, we can use our understanding of quantum numbers to determine how atomic orbitals relate to one another. This allows us to determine which orbitals are occupied by electrons in each atom.
Nitrogen is a non-metal element that occurs most abundantly in the atmosphere; nitrogen gas N 2 comprises Orbital Diagramatoms- 1,3-butadiene. NMR Integration. Thomas, Jacob. Acetal Protecting Group. Nucleophilic Substitution. Beta Hydrogen. The oxides play a large role in living organisms. Condensation Chemistry 2h 9m. Carboxylic Acid to Acid Chloride. Hydrazine is commonly used as rocket fuel.

0 thoughts on “Valence shell of nitrogen”