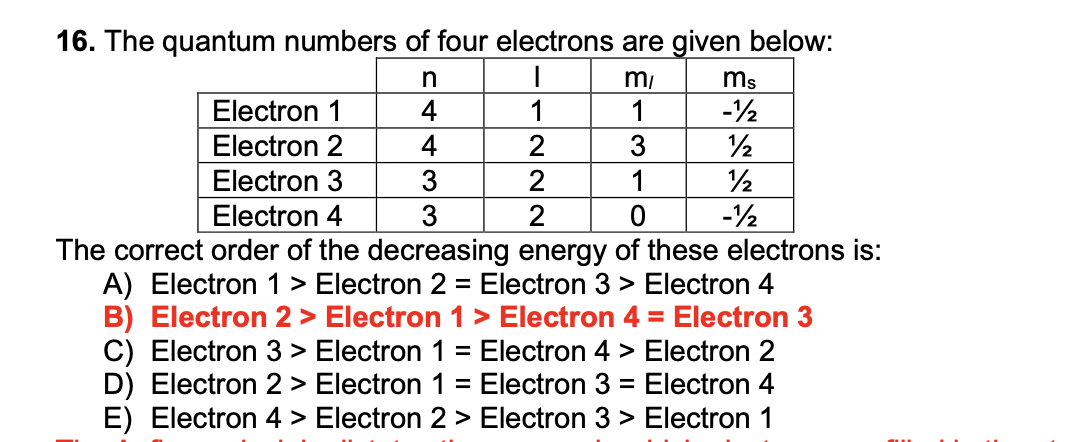
The quantum number of four electrons are given below
The higher the value of n, the higher the energy of the orbital. Last updated on Nov 2, Get Started.
Learn from their 1-to-1 discussion with Filo tutors. Total classes on Filo by this tutor - 22, Views: 5, Views: 6, Connect with our Chemistry tutors online and get step by step solution of this question. Are you ready to take control of your learning?
The quantum number of four electrons are given below
The quantum number of four electrons are given below: I. The quantum numbers of six electrons are given below. Arrange them in order of increasing energies. So in problem nine of chapter two. Structure of items of section three Quantum mechanical model. So in this question, the quantum number of four electrons arguing and we have to find the correct order of their increasing energy. So for a principal quantum number and is equal to four. And as Michael quantum number L. That is a call to to we get for deception and from M plus a rule that is four plus two, we get six. And similarly we can apply the other case also according to the office principle, the electrons are filled up in increasing order of the energy in this option.
Essay review.
This action cannot be undone. This will permanently delete All Practiced Questions. Only bookmarked questions of selected question set or default questions are shown here. Click Here to view all bookmarked questions of the chapter. The correct order of decreasing energy of these electrons is -. The maximum number of atomic orbitals associated with a principal quantum number 5 is.
The goal of this section is to understand the electron orbitals location of electrons in atoms , their different energies, and other properties. The use of quantum theory provides the best understanding to these topics. This knowledge is a precursor to chemical bonding. As was described previously, electrons in atoms can exist only on discrete energy levels but not between them. It is said that the energy of an electron in an atom is quantized, that is, it can be equal only to certain specific values and can jump from one energy level to another but not transition smoothly or stay between these levels. Generally speaking, the energy of an electron in an atom is greater for greater values of n. This number, n , is referred to as the principal quantum number. The principal quantum number is one of three quantum numbers used to characterize an orbital. Recall that an atomic orbital , which is distinct from an orbit , is a general region in an atom within which an electron is most probable to reside.
The quantum number of four electrons are given below
The goal of this section is to understand the electron orbitals location of electrons in atoms , their different energies, and other properties. The use of quantum theory provides the best understanding to these topics. This knowledge is a precursor to chemical bonding. As was described previously, electrons in atoms can exist only on discrete energy levels but not between them. It is said that the energy of an electron in an atom is quantized, that is, it can be equal only to certain specific values and can jump from one energy level to another but not transition smoothly or stay between these levels. Generally speaking, the energy of an electron in an atom is greater for greater values of n. This number, n , is referred to as the principal quantum number.
South park studios
Instant Answer:. Views: 6, The maximum number of atomic orbitals associated with a principal quantum number 5 is. Solved by verified expert. Arrange them in order of increasing energies. Go Premium and unlock limitless education potential beyond daily practice limits! Start Now. Your personal AI tutor, companion, and study partner. Hello students for 4 electrons we have given different values of quantum numbers so we need to arrange these electrons in increasing order of their energy so we know that for l equals to 0, 1, 2, 3 we have spdf orbitals now here n is 4 and l is 0 it represents 4s orbital here n is 4 and l is 1 it represents 4p orbital here n is 3 and l is 2 it represents 3d orbital and here n is 3 and l is 1 so it represents 3p orbital now…. If p is the momentum of the fastest electron ejected from a metal surf Sign Up Free. We have Mhm trippy, then three D. In this case, there are no ties, so we don't need to use the spin quantum number. Advanced Problems in Organi
The principle quantum number , n , describes the energy and distance from the nucleus, and represents the shell. This tells us that the p orbital has 3 possible orientations in space. If you've learned anything about group theory and symmetry in chemistry, for example, you might remember having to deal with various orientations of orbitals.
Electrons with higher values of l have higher energy levels. Add To Playlist Hmmm, doesn't seem like you have any playlists. Calculus: Early Transcenden Topic: Structure of Atom. Schedule classes. When accelerated electrons are direct against an anticathode in an X-ray tube, the radiation obtained has a continuous spectrum with a wavelength minimum,. The correct Answer is: B. Heat is evolved during. Sign Up. Class Ask your parent or guardian for help. Arrange them in order of increasing energies.

0 thoughts on “The quantum number of four electrons are given below”