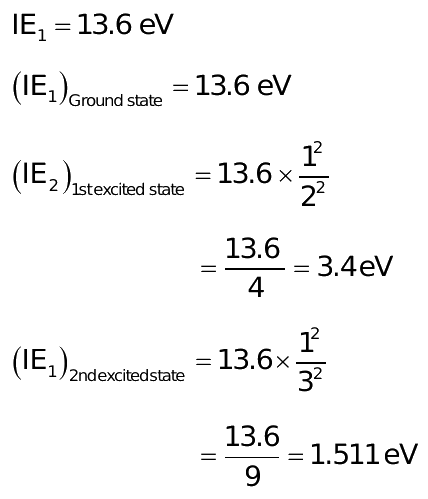
The ionization energy of hydrogen atom is 13.6
Get Started. SSC Exams.
Isotopes: The elements which have the same atomic number but different mass numbers are called isotopes. Last updated on May 25, Get Started. SSC Exams. Banking Exams. Teaching Exams.
The ionization energy of hydrogen atom is 13.6
The ionisation potential of hydrogen atom is The ionization potential of hydrogen atom is When an electron in the hydrogen atom in ground state absorb a photon of energy If ioinsation potential of hydrogen atom is The ionization energy of hydrogen atom is Hydrogen atoms in the ground state are excited by electromagnetic radiation of energy How many spectral lines will be emitted by the hydrogen atoms. What will happen if a hydrogen atom absorbs a photon of energy greater than Ionisation potential of hydrogen atom is Hydrogen atom in ground state is excited by monochromatic light of energy The spectral lines emitted by hydrogen according to Bohr's theory will be.
JEE Main.
Ionization potential of hydrogen atom is Hydrogen atoms in the ground state are excited by monochromatic radiation of photon energy The spectral lines emitted by hydrogen atoms according to Bohr's theory will be. The ionization potential of H-atom is The H-atoms in ground state are excited by mono chromatic radiations of photon energy Then the number of spectral lines emitted by the excited atoms, will be. The ionization energy of hydrogen atom is
Ionization energy, in simple terms, can be described as a measure of the difficulty in removing an electron from an atom or ion or the tendency of an atom or ion to surrender an electron. The loss of electrons usually happens in the ground state of the chemical species. Alternatively, we can also state that ionization or ionization energy is the measure of strength attractive forces by which an electron is held in a place. In more technical terms, we can describe ionization energy as the minimum energy that an electron in a gaseous atom or ion has to absorb to come out of the influence of the nucleus. It is also sometimes referred to as ionization potential and is usually an endothermic process.
The ionization energy of hydrogen atom is 13.6
The potential that an electron in a Hydrogen atom experiences results from the electrostatic interaction between the electron which is negatively charged and the proton which is positively charged. A Hydrogen nucleus is composed of a single proton. It is traditional and convenient to choose the zero level of the potential to be when the electron is extremely far away from the proton. This means that the potential energy gets more and more negative as the electron gets closer and closer to the proton.
Lvaction
Assam Rifles Tradesman. UP PGT. Maharashtra Forest Department Surveyor. CG Patwari. Allahabad High Court Group D. TS SET. Hydrogen atom in ground state is excited by a monochromatic radiation Supreme Court Junior Court Assistant. Nainital Bank PO. UP Police.
Last time, we discussed electromagnetic waves and how light is quantized as photons. We related the energy of a photon to its frequency and wavelength using the Planck-Einstein relation:.
CG Forest Guard. One electron volt is equal to :. Telangana High Court Office Subordinate. GIC Assistant Manager. Bihar Police Forest Guard. Chandigarh JBT. AOC Material Assistant. Engineering Recruitment Exams. Kolkata Police SI. MP Police Constable. Chhattisgarh AE.

I consider, that you are not right. Let's discuss it. Write to me in PM.
It do not agree
I consider, that you are not right. I am assured. Write to me in PM.