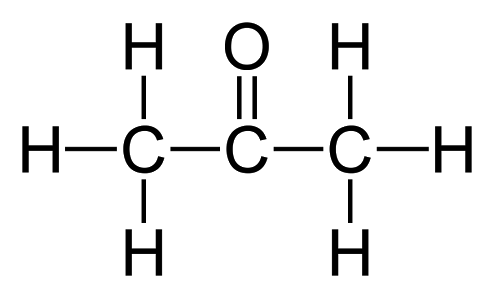
Structure of propanone
We are working on a new version of ChemSpider — if you want to try the new interface go to beta. Simple Structure Advanced History.
Acetone 2-propanone or dimethyl ketone is an organic compound with the formula CH 3 2 CO. It is a colorless, highly volatile and flammable liquid with a characteristic pungent odor. Acetone is miscible with water and serves as an important organic solvent in industry, home, and laboratory. About 6. It serves as a solvent in household products such as nail polish remover and paint thinner. Acetone is produced and disposed of in the human body through normal metabolic processes.
Structure of propanone
.
Volatile constituents of block-milk, Food Chem. Molecular shape. Acetone is produced directly or indirectly from propene.
.
We are working on a new version of ChemSpider — if you want to try the new interface go to beta. Simple Structure Advanced History. Comment on this record. Featured data source. Dimethyl formaldehy de. Dimethyl ketone. Ketone, dimethyl-.
Structure of propanone
Acetone 2-propanone or dimethyl ketone is an organic compound with the formula CH 3 2 CO. It is a colorless, highly volatile and flammable liquid with a characteristic pungent odor. Acetone is miscible with water and serves as an important organic solvent in industry, home, and laboratory. About 6. It serves as a solvent in household products such as nail polish remover and paint thinner. Acetone is produced and disposed of in the human body through normal metabolic processes. It is normally present in blood and urine. People with diabetic ketoacidosis produce it in larger amounts. From the 17th century and before modern developments in organic chemistry nomenclature , acetone was given many different names. Those names include spirit of Saturn, which was given when it was thought to be a compound of lead , and later pyro-acetic spirit and pyro-acetic ester.
Free movie screenings chicago
Retrieved July 7, Zea mays subsp. Colorless liquid with a fragrant, mint-like odor. For the musical instrument company, see Ace Tone. Heat capacity C. Archived from the original PDF on IDLH Immediate danger. Once in the atmosphere, it has a day half-life and is degraded by UV light via photolysis primarily into methane and ethane. Dairy Sci. Article Talk. Acetone occurs naturally as part of certain metabolic processes in the human body, and has been studied extensively and is believed to exhibit only slight toxicity in normal use. On 30 July , scientists reported that upon the first touchdown of the Philae lander on comet 67P 's surface, measurements by the COSAC and Ptolemy instruments revealed sixteen organic compounds , four of which were seen for the first time on a comet, including acetamide , acetone, methyl isocyanate , and propionaldehyde. In , the worldwide production capacity for acetone was estimated at 6.
Drinking methanol is harmful, not because of the CH 3 OH molecules themselves, but rather because the human body converts these molecules into methanal formaldehyde molecules by combination with oxygen:. Formaldehyde, H 2 CO, is very reactive—in the pure state it can combine explosively with itself, forming much larger molecules.
Molecules detected in outer space. The PMA type polymers of acetone would be equivalent to the product of polymerisation of propyne , except for a keto end group. Beta vulgaris subsp. Acetone 2-propanone or dimethyl ketone is an organic compound with the formula CH 3 2 CO. Comparison of determination method for volatile compounds in Thai soy sauce, Food Chem. China , 24 10 , , SRI consulting. Excipient Toxicity and Safety. USSR Engl. It arises from decarboxylation of acetoacetate.

Idea excellent, it agree with you.