Specific heat of hcl
Login to see your most recently viewed materials here. Or if you don't have an account with us yet, then click here to register.
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage. Data compilation copyright by the U. Secretary of Commerce on behalf of the U.
Specific heat of hcl
.
Jaffe, Hirshfeld, et al. Select a region with no data or click the mouse on the plot to revert to the orginal display. A, 66,
.
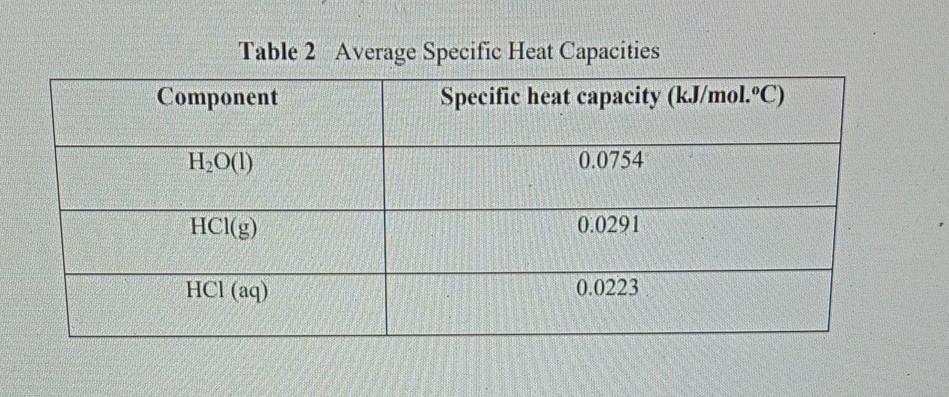
In equation form, this can be represented as the following:. That is if a constant has units, the variables must fit together in an equation that results in the same units. So C equals something with energy in the numerator and temperature in the denominator. Now, you need to use some common sense here, as we are adding heat, not work, and adding heat changes the temperature, it does not make the temperature. In words, heat capacity is the substance's ability to resist change in temperature upon exposure to a heat source. A substance with a small heat capacity cannot hold a lot of heat energy and so warms up quickly. On the other hand, a substance with a high heat capacity can absorb much more heat without its temperature drastically increasing.
Specific heat of hcl
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage. Go To: Top , References , Notes. Data compilation copyright by the U. Secretary of Commerce on behalf of the U. All rights reserved. Data compiled by: Klaus P.
Exodus trc20
The second-row diatomic hydrides AH , J. Webb and Rao, Webb, D. Dunham potential coefficients Ogilvie and Koo, London , , 73, Part I. Ghosh and Guha, Ghosh, J. Jaffe, Friedmann, et al. Quantitative scales of lewis acidities from ICR halide exchange equilibria , J. Several references are given in the list of Henry's law constants but not assigned to specific species. Price, Price, W. Ogilvie and Koo, Ogilvie, J.
Hydrochloric acid , also known as muriatic acid or spirits of salt , is an aqueous solution of hydrogen chloride HCl. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid.
Carson, Pritchard, et al. Please contact us at webmaster matweb. Self and foreign-gas line broadening Benedict, Herman, et al. Khatibi and Vu, Khatibi, P. Toth, Hunt, et al. Data compiled as indicated in comments: B - John E. Levanova, Bushneva, et al. Data, Monograph 9 , , Noren and Sunner, Noren, I. Babrov, Ameer, et al. Watanabe, Nakayama, et al. Your institution may already be a subscriber.

Improbably!
I have removed this phrase
I well understand it. I can help with the question decision.