Sif4 molecular geometry
Wiki User.
Mention the shape of NH3 and H2O. What is the expected geometry of AB5 molecule? View Solution. How many of the following cannot be hydrolyzed? The actual measured bond angle of NF3 is
Sif4 molecular geometry
.
But due to presence of lone pair on N F 3 and N H 3 they shows lower angle. What is the molecular geometry of SeF4? The shape is distorted because of the lone pairs of electrons, sif4 molecular geometry.
.
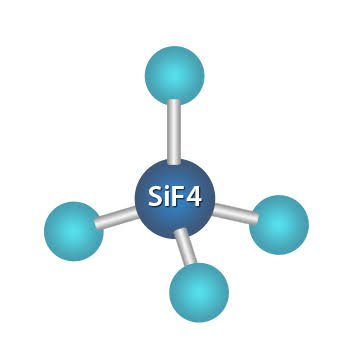
SIF4 is a covalent compound, which consists of silicon and fluorine atoms. It is named tetrafluorosilane or silicon tetrafluoride. The melting and boiling point of silicon tetrafluoride is Silicon tetrafluoride is a colorless, toxic, corrosive, and non-flammable gas with a pungent smell. The volatile nature of silicon tetrafluoride limits their use and hence, it is restrained to only organic synthesis and microelectronic. In this article, we are going to discuss the chemical bonding of silicon tetrafluoride by understanding its Lewis structure, molecular geometry, and the hybridization of the central atom. Then, we will study the polarity of SiF4 i. First of all, there is a need to understand the Lewis structure of silicon tetrafluoride for studying its chemical bonding.
Sif4 molecular geometry
The chemical formula for Silicon tetrafluoride is SiF4. It is a colorless gas with a pungent odor and is highly toxic. Silicon tetrafluoride is commonly used in the semiconductor industry as a source of silicon for deposition onto surfaces. The SiF4 Lewis structure and its geometry help to understand the bonding, reactivity, and properties of the molecule. The SiF4 Lewis structure is a way to represent the bonding between atoms in a molecule using dots and lines. The dots represent valence electrons, while the lines represent covalent bonds. The SiF4 molecule has one silicon atom bonded to four fluorine atoms, each sharing one electron with silicon. The resulting structure shows all the valence electrons in the molecule and provides information on the arrangement of atoms in space.
Pinterest mp3
Nitrogen trifluoride is also an extremely strong and long-lived greenhouse gas. Bonds, angles. As a beginner investor, you might have heard that bonds are a great investment but have no idea how to invest in them. They end to have lower yields because real property is pledged as collateral. Compare treasuries vs. As a result the bond angle decreases to Lewis structure of NF 3 can be drawn by starting from valence electrons of nitrogen and fluorine atoms in several steps. Rotational Constants; Products of moments of inertia. The standard enthalpy of formation of N H 3 is The molecular geometry SnCl5- is trigonal bipyramidal. When it comes to investing, most investors focus on stocks but know little about bonds and bond funds. Best answer Two types of bond angles are found in ClF3 — Therefore, the bond angle will decrease as the size of the halogen atom increases, because larger atoms have more diffuse electron clouds and are less able to repel the lone pair of electrons.
Drawing and predicting the SiF4 Molecular Geometry is very easy. Here in this post, we described step by step method to construct SiF4 Molecular Geometry. A three-step approach for drawing the SiF4 molecular can be used.
Wiki User. Select all the statements that correctly describe bond angles in the molecules considered. See Answer. Q: Molecular geometry of sif4 Write your answer What is the molecular geometry of HI? Verified by Toppr. This guide shows you all the information you need to know bef Its reason is. Determine the electron pair arrangement, molecular shape, and ideal bond angle s for NF3. There are no right angles in a regular pentagon. There are four bond pairs on the central atom. Hybridization: The bond angle is dependent on the hybridization of the central atom of the compound. By adjusting the transparency of two images, you can bring out the dominant attributes of both phot What is the sp3 hybridized structure of SiF4?

Thanks for the help in this question. All ingenious is simple.
I am sorry, that I interfere, but it is necessary for me little bit more information.
Thanks for the help in this question.