Sf4 bond angle
What is the shape of SF 4 including bond angles? The formula used to calculate the hybridization of a molecule is as follows:.
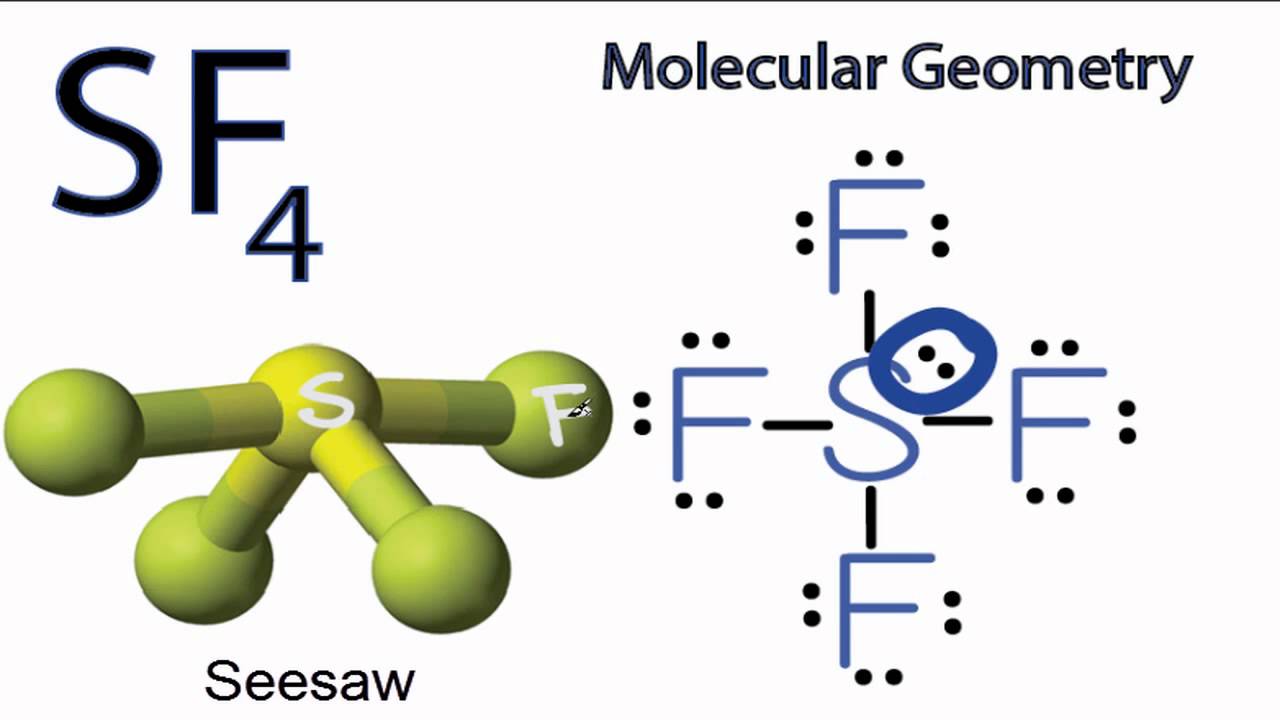
The hybridization that is involved in SF 4 is sp 3 d type. Here will learn and understand how to determine SF 4 hybridization. We will discuss the steps in detail. In order to determine the hybridization of sulphur tetrafluoride, you have to first understand its Lewis structure and the number of valence electrons that are present. The SF 4 molecule consists of a total of 34 valence electrons.
Sf4 bond angle
Hybridization of SF4 is sp 3 d. In this hybridization, a total of five hybrid orbitals are formed. Sulfur tetrafluoride, or SF4 is a chemical compound made up of four fluorine atoms and one sulfur atom. It is a colorless compound that releases poisonous HF gas when reacts with water or moisture. In this article, we will learn about the hybridization of SF4 and its geometry with a brief introduction to the SF4 molecule and the hybridization process. Sulfur Tetrafluoride is a gaseous compound that consists of one sulfur atom and is bonded to four fluorine atoms. It is a colorless compound and when reacts with water or moisture releases Hydrogen Fluoride gas. The molecular compound SF4 is well-known for its unique characteristics. In this hybridization, one s orbital, three p orbitals and one d orbital of third shell participate in creating five hybrid orbitals. In hybridization of SF4, sulphur is the central atom which is bonded to four flourine atoms. Sulfur has 6 valence electrons.
Read full.
The process of mixing of atomic orbitals belonging to the same atom of slightly different energies so that a redistribution of energy takes place between them resulting in the formation of new sets of orbitals of equivalent energies and shape is called hybridization. The new orbitals in this form are known as hybrid orbitals. Like pure orbitals the hybrid orbitals are used in Bond formation. Hybridization is a hypothetical concept and has been introduced in order to explain the characteristic geometrical shapes of polyatomic molecules. The central atom is S. So, to explain in simple terms, its bonding regions are four having one lone pair.
Sulfur tetrafluoride is the chemical compound with the formula S F 4. It is a colorless corrosive gas that releases dangerous HF upon exposure to water or moisture. Despite these unwelcome characteristics, this compound is a useful reagent for the preparation of organofluorine compounds , [3] some of which are important in the pharmaceutical and specialty chemical industries. Of sulfur's total of six valence electrons , two form a lone pair. One of the three equatorial positions is occupied by a nonbonding lone pair of electrons. Consequently, the molecule has two distinct types of F ligands, two axial and two equatorial. It is typical for the axial ligands in hypervalent molecules to be bonded less strongly.
Sf4 bond angle
The Lewis electron-pair approach can be used to predict the number and types of bonds between the atoms in a substance, and it indicates which atoms have lone pairs of electrons. This approach gives no information about the actual arrangement of atoms in space, however. Keep in mind, however, that the VSEPR model, like any model, is a limited representation of reality; the model provides no information about bond lengths or the presence of multiple bonds. The VSEPR model can predict the structure of nearly any molecule or polyatomic ion in which the central atom is a nonmetal, as well as the structures of many molecules and polyatomic ions with a central metal atom.
London marathon 2018 full race
In comparison, the other three fluorines are equatorial. Like Article Like. Molecular Orbital Theory. Related questions How do I determine the bond angle in a molecule? Formation of Complexes. You can reuse this answer Creative Commons License. This article is being improved by another user right now. But hurry up, because the offer is ending on 29th Feb! Admission Experiences. These valence electrons are arranged in 3s 2 3p 4. Hence the molecular geometry will be a see-saw. Learn more topics related to Chemistry. As a result, we can identify five distinct electron density zones.
The Lewis electron-pair approach can be used to predict the number and types of bonds between the atoms in a substance, and it indicates which atoms have lone pairs of electrons. This approach gives no information about the actual arrangement of atoms in space, however.
Truong-Son N. What is hybridization? These atoms form a trigonal bipyramidal shape. Vote for difficulty :. What is the shape of SF4 including bond angles? Download Important Formulas pdf. Access more than. It will also help in determining the hybrid orbitals count used by the atom by knowing the steric number. What is SF4's electrical geometry? Post My Comment. Biological Activity.

0 thoughts on “Sf4 bond angle”