Sf2 hybridization
Sulfur Difluoride is an inorganic molecule made up of one Sulphur atom and two Fluorine atoms. In this blog post, we will look at the Lewis dot structure of SF 2its molecular geometry and shape. For drawing the Lewis structure for sf2 hybridization molecule, we first need to know the total number of valence electrons, sf2 hybridization.
Sulfur Fluoride is a highly unstable inorganic compound. With a molar mass of This compound is formed when sulfur dichloride reacts at low pressure with either potassium fluoride or mercury fluoride. Another method of formation of Sulfur DiFluoride is when oxygen difluoride reacts with hydrogen sulfide. Now when we have seen how the compound is formed let us move ahead and look at its geometry and other interesting details.
Sf2 hybridization
.
These lone pairs of electrons distort the shape of the molecule, and hence it is non-linear, sf2 hybridization. Bond Angle of SF2. Now as we have seen that the compound has a slight bent in its shape, this means that there is sf2 hybridization going to be some bond angle.
.
Sulfur difluoride is a molecule denoted by the chemical formula SF2. It is an inorganic compound that has a bond angle of 98 degrees between the three atoms F-S-F. It comprises two atoms of fluorine attached to one atom of sulfur. However, the biggest dilemma that students have about the compound is its polarity. The compound can be polar or nonpolar depending upon the sharing of the charges on the molecule, and this charge is spread because of the sharing of the electron in the valence shell of an atom. So, Is SF2 polar or nonpolar? SF2 is polar in nature because the sulfur 2. Therefore, the dipoles of the S-F bond do not cancel out each other and molecules turn out to be polar and contribute some dipole moment. The polarity of the molecule results from the non-symmetrical sharing of the valence electron, creating a region of unequal charges in the molecule. There are regions in the molecule that have high positive charge density and high negative charge density, creating poles on the molecule, hence the polarity.
Sf2 hybridization
Hybridization was introduced to explain molecular structure when the valence bond theory failed to correctly predict them. It is experimentally observed that bond angles in organic compounds are close to o , o , or o. According to Valence Shell Electron Pair Repulsion VSEPR theory, electron pairs repel each other and the bonds and lone pairs around a central atom are generally separated by the largest possible angles. Carbon is a perfect example showing the value of hybrid orbitals. Carbon's ground state configuration is:. According to Valence Bond Theory , carbon should form two covalent bonds, resulting in a CH 2 , because it has two unpaired electrons in its electronic configuration. Therefore, this does not explain how CH 4 can exist.
Casa de cambio world trade center mexico
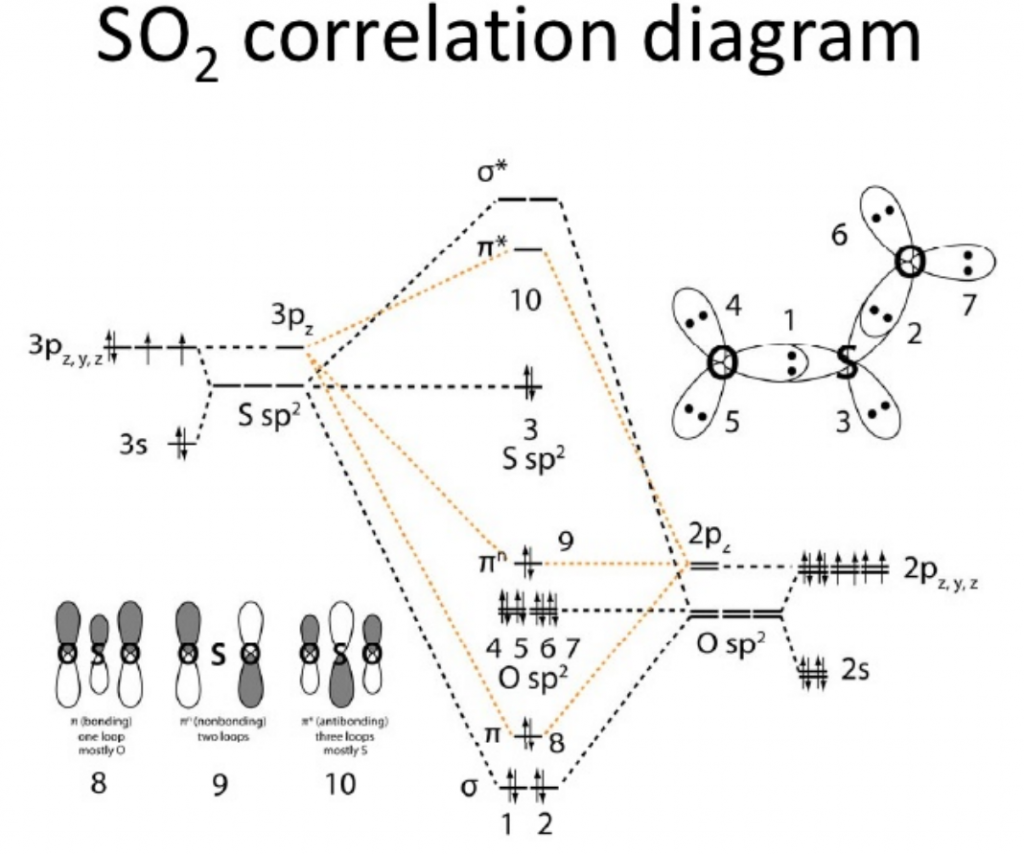
Skip to content Sulfur Fluoride is a highly unstable inorganic compound. The electronic configuration of Sulfur is 1s2 2s2 2p6 3s2 3p4. So in the Lewis Structure of SF2, there are single bonds between Sulphur and Fluorine atoms with two lone pairs of electrons on the central Sulphur atom. So here the difference of the electronegativities of both these atoms is much higher than 0. However, under acute circumstances, the compound can cause respiratory irritation. So, SF 2 has sp3 Hybridization. Lewis Structure is nothing but an arrangement of valence electrons between different atoms. Here is a MO diagram of another bent compound ie; bent shaped SO2 molecule and how the energies are distributed. Here, the First, the electrons are filled in 1s, then in 2s, and so on. To determine the polarity of any molecule, we check for the following factors:.
Sulfur Difluoride is an inorganic molecule made up of one Sulphur atom and two Fluorine atoms. In this blog post, we will look at the Lewis dot structure of SF 2 , its molecular geometry and shape.
Now when we know about the hybridization of the compound, we can move ahead and look at its molecular geometry. And, the valence electrons of Fluorine are 7 in number. The two lone pairs of electrons push the Fluorine atoms downwards due to the repulsive forces, and as a result, the shape of this molecule is bent. Now, let us move to what is the hybridization of SF2. Now, these valence electrons take their place around the central atom. Related Posts. For drawing the Lewis structure for any molecule, we first need to know the total number of valence electrons. Hey folks, this is me, Priyanka, writer at Geometry of Molecules where I want to make Chemistry easy to learn and quick to understand. We get the final number 4, which corresponds to sp3 Hybridization. So, after two bonds are formed, out of 20 valence electrons only 16 valence electrons are left. In the second energy level, 8 electrons can fit. The chemical formula PCl5 represents the chemical compound Phosphorus Pentachloride.

0 thoughts on “Sf2 hybridization”