Scl2 lewis structure
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter.
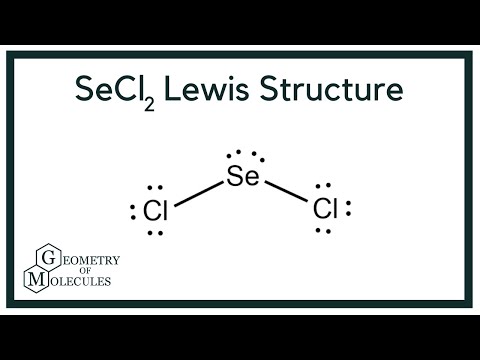
Sulfur dichloride SCl 2 contains one sulfur atom and two chlorine atoms. Lewis structure of SCl 2 contains only two S-Cl bonds. There are two lone pairs on sulfur atom and three lone pairs on each chlorine atom in SCl 2 lewis structure. Both chlorine atoms have made single bonds with sulfur atom. Also, there are three lone pairs exist on both chlorine atoms and two lone pairs on sulfur atom.
Scl2 lewis structure
.
Naming Other Substituents. Alkane Reactions. Heat Capacity.
.
There are 2 single bonds between the Sulfur atom S and each Chlorine atom Cl. There are 2 lone pairs on the Sulfur atom S and 3 lone pairs on both the Chlorine atoms Cl. In order to find the total valence electrons in SCl2 sulfur dichloride molecule , first of all you should know the valence electrons present in sulfur atom as well as chlorine atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Sulfur is a group 16 element on the periodic table. Chlorine is group 17 element on the periodic table. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center.
Scl2 lewis structure
Ready to learn how to draw the lewis structure of SCl2? Here, I have explained 6 simple steps to draw the lewis dot structure of SCl2 along with images. The Sulfur atom S is at the center and it is surrounded by 2 Chlorine atoms Cl. The Sulfur atom has 2 lone pairs and both the Chlorine atoms have 3 lone pairs. Note: Take a pen and paper with you and try to draw this lewis structure along with me.
Bro3 oxidation number
When we draw lewis structures, there are several guidelines to follow. Thermochemical Equations. Periodic Properties of the Elements 2h 57m. Periodic Trend: Successive Ionization Energies. The Alkyl Groups. Number of steps can be changed according the complexity of the molecule or ion. Periodic Trend: Cumulative. Standard Temperature and Pressure. The Electron Configuration: Condensed. Alcohol Reactions: Dehydration Reactions. Balancing Chemical Equations. Multiplication and Division Operations. The Electron Configuration: Quantum Numbers. Classification of Ligands.
Sulfur dichloride is a red viscous liquid at room temperature. It has a pungent chlorine-like odor. It reacts with water to form chlorine-containing acids.
Periodic Table: Phases. Hybridization Concept 1. Resonance Structures. Hydrohalogenation Reactions. Total electron pairs are determined by dividing the number total valence electrons by two. Collision Theory. Group 1A and 2A Reactions. Molecular Geometry. Cell Potential: Standard. Laboratory Materials.

I am final, I am sorry, but it does not approach me. There are other variants?
I consider, that you are mistaken. I can defend the position. Write to me in PM, we will talk.
It is a pity, that now I can not express - I am late for a meeting. I will be released - I will necessarily express the opinion.