Prodrugs
Thank you for visiting nature.
Federal government websites often end in. The site is secure. Prodrugs are bioreversible, inactive drug derivatives, which have the ability to convert into a parent drug in the body. In the past, prodrugs were used as a last option; however, nowadays, prodrugs are considered already in the early stages of drug development. Optimal prodrug needs to have effective absorption, distribution, metabolism, and elimination ADME features to be chemically stable, to be selective towards the particular site in the body, and to have appropriate safety.
Prodrugs
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Prodrugs are molecules with little or no pharmacological activity that are converted to the active parent drug in vivo by enzymatic or chemical reactions or by a combination of the two. Prodrugs have evolved from being serendipitously discovered or used as a salvage effort to being intentionally designed. Such efforts can avoid drug development challenges that limit formulation options or result in unacceptable biopharmaceutical or pharmacokinetic performance, or poor targeting. In this Review, we highlight prodrug design strategies for improved formulation and pharmacokinetic and targeting properties, with a focus on the most recently marketed prodrugs. We also discuss preclinical and clinical challenges and considerations in prodrug design and development. This is a preview of subscription content, access via your institution. Stella, V. Prodrugs: Some thoughts and current issues. Clas, S.
Acta Pharm.
Prodrugs are bioreversible, inactive drug derivatives, which have the ability to convert into a parent drug in the body. In the past, prodrugs were used as a last option; however, nowadays, prodrugs are considered already in the early stages of drug development. Optimal prodrug needs to have effective absorption, distribution, metabolism, and elimination ADME features to be chemically stable, to be selective towards the particular site in the body, and to have appropriate safety. Here, we present recently investigated prodrugs, their pharmaceutical and clinical advantages, and challenges facing the overall prodrug development. Given examples illustrate that prodrugs can accomplish appropriate solubility, increase permeability, provide site-specific targeting i.
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. The development of prodrugs is presently well established as a strategy for improving the physicochemical, biopharmaceutical or pharmacokinetic properties of pharmacologically potent compounds and thereby overcoming barriers to a drug's developability and usefulness. Clinically, the majority of prodrugs are used with the aim of enhancing drug permeation by increasing drug lipophilicity and more recently to improve drug water solubility. This Review provides an overview of functional groups that are amenable to prodrug design, and highlights major applications of the prodrug strategy, including improving oral absorption, improving aqueous solubility, enhancing lipophilicity, enhancing active transport as well as achieving site-selective delivery. In both drug discovery and development, prodrugs have become an established tool for improving physicochemical, biopharmaceutical or pharmacokinetic properties of pharmacologically active agents.
Prodrugs
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Prodrugs are molecules with little or no pharmacological activity that are converted to the active parent drug in vivo by enzymatic or chemical reactions or by a combination of the two. Prodrugs have evolved from being serendipitously discovered or used as a salvage effort to being intentionally designed.
Top restaurants in sorrento
Directed enzyme prodrug therapies. Sharma, S. Oseltamivir: a clinical and pharmacological perspective. Activation of benznidazole by trypanosomal type I nitroreductases results in glyoxal formation. Both major types can be further categorized into subtypes, based on factors such as Type I whether the intracellular bioactivation location is also the site of therapeutic action, or Type 2 whether or not bioactivation occurs in the gastrointestinal fluids or in the circulation system. Bueno, A. Archived at the Wayback Machine June The prodrug approach is used for the optimization of newly discovered chemical entities, as well as to improve the properties of already marketed drugs. Note 1 : Modified from ref. Yang, C. Google Scholar McComb, R. Read Edit View history.
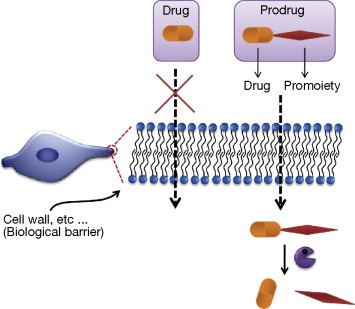
A prodrug is a pharmacologically inactive medication or compound that, after intake , is metabolized i. Prodrugs are often designed to improve bioavailability when a drug itself is poorly absorbed from the gastrointestinal tract.
Baltzer, B. Cancer 81 , 99— Ben-Menachem E. Enhancement of transdermal delivery of 6-b-naltrexol via a codrug linked to hydroxybupropion. Beaumont, K. Dando, T. Zhang, X. Niculescu-Duvaz D. The prodrug approach is used for the optimization of newly discovered chemical entities, as well as to improve the properties of already marketed drugs. Design, synthesis, enzymatic conversion to gabapentin, and transport by intestinal solute transporters. Increasing knowledge of enzymes and membrane transporters in terms of structure, specificity toward different substrates, mechanism of action, and role can lead to the future development of successful targeted prodrug delivery. Part 2 eds Stella, V. Aljuffali, I. Learn more.

Completely I share your opinion. It seems to me it is excellent idea. I agree with you.
Has found a site with interesting you a question.
I with you do not agree