Phospholipase a2
Inflammation and Regeneration volume 36Article number: 7 Cite this article. Metrics details.
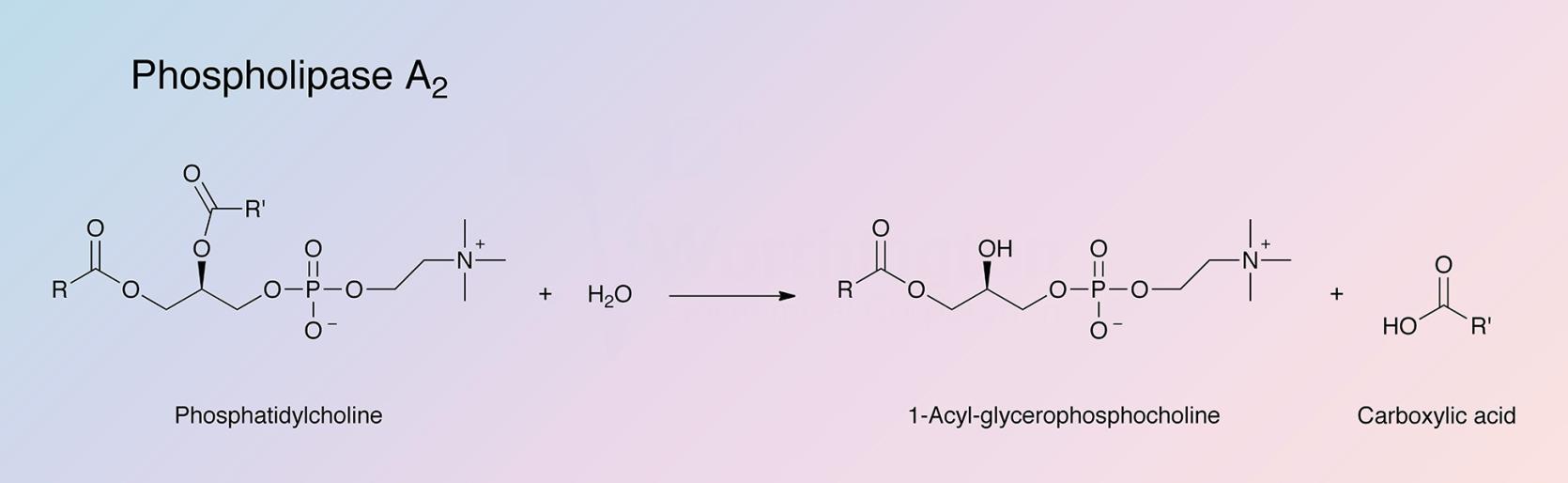
Federal government websites often end in. The site is secure. The phospholipase A 2 PLA 2 superfamily consists of many different groups of enzymes that catalyze the hydrolysis of the sn-2 ester bond in a variety of different phospholipids. The products of this reaction, a free fatty acid, and lysophospholipid have many different important physiological roles. This review focuses on the superfamily of PLA 2 enzymes, and then uses three specific examples of these enzymes to examine the differing biochemistry of the three main types of these enzymes. PLA 2 s form a superfamily that currently contains fifteen separate, identifiable groups and numerous subgroups of PLA 2 [ 1 — 3 ]. These enzymes are characterized by their ability to specifically hydrolyze the sn-2 ester bond of phospholipid substrate as shown in Fig.
Phospholipase a2
The enzyme phospholipase A 2 EC 3. This particular phospholipase specifically recognizes the sn 2 acyl bond of phospholipids and catalytically hydrolyzes the bond, releasing arachidonic acid and lysophosphatidic acid. Upon downstream modification by cyclooxygenases or lipoxygenases , arachidonic acid is modified into active compounds called eicosanoids. Eicosanoids include prostaglandins and leukotrienes , which are categorized as anti-inflammatory and inflammatory mediators. PLA2 enzymes are commonly found in mammalian tissues as well as arachnid, insect, and snake venom. Due to the increased presence and activity of PLA2 resulting from a snake or insect bite, arachidonic acid is released from the phospholipid membrane disproportionately. As a result, inflammation and pain occur at the site. Additional types of phospholipases include phospholipase A 1 , phospholipase B , phospholipase C , and phospholipase D. Phospholipases A 2 include several unrelated protein families with common enzymatic activity. Two most notable families are secreted and cytosolic phospholipases A 2.
Biochemistry and physiology of mammalian secreted phospholipases A 2. Deletion of secretory group V phospholipase A 2 attenuates cell migration and airway hyperresponsiveness in immunosensitized mice. Background Phospholipase A 2 PLA 2 is a group of enzymes that hydrolyze phospholipids to phospholipase a2 fatty acids and lysophospholipids Fig, phospholipase a2.
.
Federal government websites often end in. The site is secure. The data presented in this review were compiled from the cited work. Details about the adapted figures and graphic are available upon request. The phospholipase A2 PLA2 superfamily of phospholipase enzymes hydrolyzes the ester bond at the sn-2 position of the phospholipids, generating a free fatty acid and a lysophospholipid. The superfamily of PLA2 comprises at least six big families of isoenzymes, based on their structure, location, substrate specificity and physiologic roles. Phospholipase A2 PLA2 belongs to the lipolytic family of enzymes that hydrolyze the ester bond at the sn-2 position of the phospholipids.
Phospholipase a2
The enzyme phospholipase A 2 EC 3. This particular phospholipase specifically recognizes the sn 2 acyl bond of phospholipids and catalytically hydrolyzes the bond, releasing arachidonic acid and lysophosphatidic acid. Upon downstream modification by cyclooxygenases or lipoxygenases , arachidonic acid is modified into active compounds called eicosanoids.
150 foot pounds to nm
Structures of free and inhibited human secretory phospholipase A2 from inflammatory exudate. Clin Chim Acta. Short-chain phosphatidylethanolamines: physical properties and susceptibility of the monomers to phospholipase A2 action. Biology of Secretory Phospholipase A2. Analysis of expression of secreted phospholipases A2 in mouse tissues at protein and mRNA levels. When phosphorylation is coupled with an influx of calcium ions, cPLA2 becomes stimulated and can translocate to the membrane to begin catalysis. The crystal structure of phospholipase A 2 from Indian cobra reveals a novel trimeric association. These phospholipases are involved in cell signaling processes, such as inflammatory response. Abstract Within the phospholipase A 2 PLA 2 superfamily that hydrolyzes phospholipids to yield fatty acids and lysophospholipids, the secreted PLA 2 sPLA 2 enzymes comprise the largest family that contains 11 isoforms in mammals. Layer of lipid phosphates - yellow dots. In addition, sPLA 2 -IIA is abundantly expressed in the human and rat adipose tissues in obesity and the pharmacological inhibition of this isoform attenuates the adipose tissue inflammation in rats [ 18 , 52 ]. Prog Lipid Res.
Federal government websites often end in. Before sharing sensitive information, make sure you're on a federal government site. The site is secure.
Kumoanogoh University of Osaka, Japan for providing an opportunity to write this manuscript. Group IV cytosolic phospholipase A2 binds with high affinity and specificity to phosphatidylinositol 4,5-bisphosphate resulting in dramatic increases in activity. Regulation of lysophospholipase activity of the kDa phospholipase A2 and activation in mouse peritoneal macrophages. Phospholipase A2 Phospholipase C Diacylglycerol lipase. Nature Medicine. Ceramide 1-phosphate acts as a positive allosteric activator of group IVA cytosolic phospholipase A 2 alpha and enhances the interaction of the enzyme with phosphatidylcholine. Anti-inflammatory properties of a platelet-activating factor acetylhydrolase. KEGG entry. As a library, NLM provides access to scientific literature. Interfacial catalysis: the mechanism of phospholipase A2. On the other hand, we have shown that sPLA 2 -X is expressed abundantly in GI-lining cells and participates in phospholipid digestion [ 19 ]. Membrane recognition by phospholipid-binding domains. Conclusions It is now obvious that at least four sPLA 2 s are involved in metabolic regulation through distinct mechanisms, as summarized below.

I regret, that, I can help nothing, but it is assured, that to you will help to find the correct decision.
You have quickly thought up such matchless phrase?