Ph3 polarity
Pages: [ 1 ] Go Down, ph3 polarity. Read times. Why is PH3 a polar molecule when P and H have the same electronegativity? Is it because of the lone pair on P, making electron density greater on P?
Phosphine has a trigonal pyramidal structure, similar to that of phosphorus. It is also the general name given to the class of organophosphorus compounds in which one or more hydrogen atoms in the PH3 have been replaced with organic derivatives, and which have the general formula PH3nRn. Phosphine IUPAC name: phosphane is a colourless, combustible, and highly poisonous molecule with the chemical formula PH 3 , which is classified as a pnictogen hydride. However, because of the presence of substituted phosphine and diphosphine in technical-grade samples, they have a strong rotting fish aroma that is extremely unpleasant to the nose and throat P 2 H 4. Because of the presence of P 2 H 4 , PH 3 is spontaneously combustible in the air pyrophoric , resulting in the production of a very bright flame. Phosphine is an extremely poisonous respiratory toxin that is immediately lethal at concentrations of 50 parts per million ppm. Phosphine has a trigonal pyramidal shape throughout its whole chemical structure.
Ph3 polarity
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter. Chemical Properties. Physical Properties. Intensive vs. Extensive Properties. Scientific Notation. Metric Prefixes. Significant Figures. Significant Figures: Precision in Measurements. Significant Figures: In Calculations. Conversion Factors.
How is PH3 developed? Hydrohalogenation Reactions.
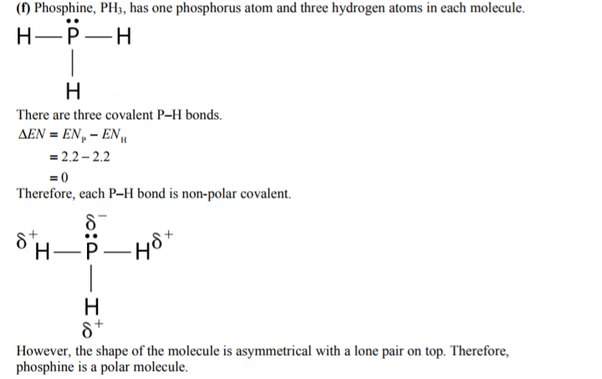
PH3 is one of the most confusing chemical compounds in Chemistry because of its polarity that can be polar and nonpolar. The Phosphine or Phosphorus Trihydride is a toxic flammable gas that smells like rotten fish. Phosphorus Trihydrate is a polar molecule since it has a lone electron with electron-electron repulsion that results in a bent structure. The Phosphorus atom and Hydrogen atom bond is nonpolar because of the same electronegativity; however, Phosphorus has an unshared pair of electrons. In addition, PH3 is considered polar because the chemical compound has three Hydrogen atoms and five valence electrons.
Whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. Electronegativity is a measure of the tendency of an atom to attract electrons or electron density towards itself. It determines how the shared electrons are distributed between the two atoms in a bond. The more strongly an atom attracts the electrons in its bonds, the larger its electronegativity. Electrons in a polar covalent bond are shifted toward the more electronegative atom; thus, the more electronegative atom is the one with the partial negative charge. The greater the difference in electronegativity, the more polarized the electron distribution and the larger the partial charges of the atoms. In general, electronegativity increases from left to right across a period in the periodic table and decreases down a group. Metals tend to be less electronegative elements, and the group 1 metals have the lowest electronegativities.
Ph3 polarity
Improve your experience by picking them. Skip to main content. Select textbook and university Improve your experience by picking them. Table of contents. Intro to General Chemistry 3h 51m.
The upper floor porn
Coordination Complexes. Balancing Redox Reactions: Basic Solutions. Structure of Phosphine Phosphine has a trigonal pyramidal shape throughout its whole chemical structure. Molecular Polarity Concept 1. Intermolecular Forces. Significant Figures: Precision in Measurements. This works but chemists use different methods to determine solubilities. Phosphine is a chemical The larger electronegativity difference makes a larger dipole moment. Reaction Quotient.
The ability of an atom in a molecule to attract shared electrons is called electronegativity.
Alcohol Reactions: Oxidation Reactions. Lewis Dot Structures: Ions. Vapor Pressure Lowering Raoult's Law. Periodic Properties of the Elements 2h 57m. Your email address will not be published. Is there slightly greater electron density towards the P or the Hs? Chemical Kinetics 2h 43m. Dimensional Analysis. NEET Syllabus When PH 3 interacts with hydrogen iodide, it behaves as a Lewis base by donating its lone pair of electrons to the reaction. The two atoms do not belong to the same group within the periodic table, and with this, the PH3 has three Hydrogen and five valence electrons in its atom. Why is PH3 a polar molecule when P and H have the same electronegativity? So after thinking about it a bit, and from the Wikipedia info, the following are my thoughts. Molecular Polarity Concept 1.

I firmly convinced, that you are not right. Time will show.