Pf5 lewis structure
Q: Qa
What shapes do you predict for these two molecules? What is the hybridization for the nitrogen in each molecule? Therefore, there are eight valence electrons in total. The Lewis structure shows that nitrogen has one lone pair and is bonded to three fluorine atoms. To explain the stability of NF3, we need to Ask your question!
Pf5 lewis structure
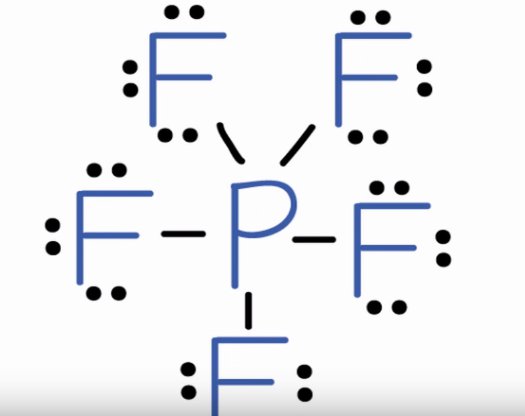
Views: 5, Connect with our Chemistry tutors online and get step by step solution of this question. Are you ready to take control of your learning? American National Curriculum. High School. All topics. The Lewis structure for P F 5. Question asked by Filo student. Views: 5, students. Step 2: The Lewis structure represents the arrangement of atoms and valence electrons in a molecule. Step 3: The first step in drawing the Lewis structure of PF5 is to determine the total number of valence electrons. Step 4: Phosphorus P is in Group 5A, so it has 5 valence electrons. Fluorine F is in Group 7A, so it has 7 valence electrons. Step 6: Next, we determine the central atom by identifying the atom with the lowest electronegativity. In PF5, phosphorus P is the central atom.
A: In the first reaction, the given carbonyl compound reacts with hydrogen in the presence of a….
Phosphorus pentafluoride , P F 5 , is a phosphorus halide. It is a colourless, toxic gas that fumes in air. Phosphorus pentafluoride was first prepared in by the fluorination of phosphorus pentachloride using arsenic trifluoride , which remains a favored method: [1]. Phosphorus pentafluoride can be prepared by direct combination of phosphorus and fluorine :. Single-crystal X-ray studies indicate that the PF 5 has trigonal bipyramidal geometry.
Data compilation copyright by the U. Secretary of Commerce on behalf of the U. All rights reserved. View table. Lias, Rhoda D. Levin, and Sherif A. Lias, John E. Bartmess, Joel F. Liebman, John L.
Pf5 lewis structure
Transcript: Hi, this is Dr. Let's do the Lewis structure for PF5. On the periodic table, Phosphorus is in group 5, it has 5 valence electrons. Fluorine, group 7, but we have five of those, so we need to multiply that 7 by 5. Five plus 40 valence electrons. We'll put the Phosphorus in the center, and then the Fluorines, we have five of them, let's put them around it like this. We'll connect the Phosphorus to each Fluorine with a single line representing a pair of electrons, like that.
Best alternative songs 2017
In PF5, phosphorus P is the central atom. Write your answer in Scientific… A:. Other anions. CuF CuF 2. B The formal oxidation number of the silicon atom. Explain why two different types of bonding are observed. Green Yellow Orange Red A:. Views: 5, students. ErF 3. We have 30 valence electrons. Related Questions Q:. N verify what is Y N?
The PF5 Lewis structure refers to the arrangement of atoms and electrons in a molecule of phosphorus pentafluoride PF5.
RaF 2. Draw the Lewis structure for the phosphorus pentafluoride PF molecule. If you wanted to make sure you had the right structure, you could check the formal charges and you'd see that they're all zero. Q: How many electrons in an atom could have these sets of quantum numbers? Draw the Lewis structure for phosphorus pentachloride, please include the following. On the periodic table, Phosphorus is in group 5, it has 5 valence electrons. Using the following data, determine…. LaF 3. Draw the Lewis Structure for PH3: Now answer the following questions based on your Lewis structure: Enter an integer value only: of bonding electrons of non bonding electrons of lone pairs on the phosphorus atom. Phosphorus pentafluoride can be prepared by direct combination of phosphorus and fluorine :.

I apologise, but, in my opinion, you are mistaken. Let's discuss. Write to me in PM, we will communicate.