Pcl3 electron domain geometry
When we talk about the hybridization of PCl 3 students should not confuse it with PCl5. PCl 3 is sp 3 hybridized.
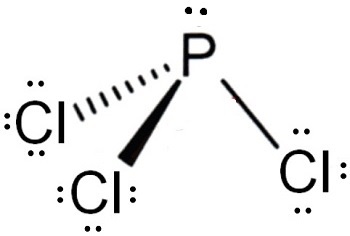
The Lewis structure of PCl3 consists of a central phosphorus atom P and three external chlorine atoms Cl. There are three single bonds between the phosphorus atom P and each of the chlorine atoms Cl. There is one lone pair of electrons on the phosphorus atom P and three lone pairs of electrons on each of the chlorine atoms Cl. The PCl3 Lewis structure is shown below:. Phosphorus and chlorine are elements of group 15 and 17 of the periodic table, respectively.
Pcl3 electron domain geometry
.
During the excited stated one of the s electrons moves to an empty d orbital resulting in the change in electronic configuration. The bond angles between the central phosphorus atom and the three chlorine atoms are approximately
.
Phosphorus trichloride is made up of one Phosphorus atom and three Chlorine atoms, having a chemical formula of PCl3. It is a volatile liquid that reacts with water and releases HCl gas. It is a toxic compound but is used in several industries. Phosphorus Trichloride is widely used in manufacturing Phosphites and other organophosphorus compounds. One needs to know the total number of valence electrons for a molecule to construct the Lewis Dot Structure. To calculate the total number of valence electrons of this molecule, we will add up the valence electrons of both Phosphorus and Chlorine atoms. Chlorine has seven valence electrons, but as there are three atoms of Chlorine, we will multiply this number by 3.
Pcl3 electron domain geometry
We begin by assuming a Lewis structure model for chemical bonding based on valence shell electron pair sharing and the octet rule. We thus assume the nuclear structure of the atom, and we further assume the existence of a valence shell of electrons in each atom which dominates the chemical behavior of that atom. A covalent chemical bond is formed when the two bonded atoms share a pair of valence shell electrons between them. We know that double bonds are generally stronger and have shorter lengths than single bonds, and triple bonds are stronger and shorter than double bonds. We should expect that the properties of molecules, and correspondingly the substances which they comprise, should depend on the details of the structure and bonding in these molecules. The relationship between bonding, structure, and properties is comparatively simple in diatomic molecules, which contain two atoms only, e. A polyatomic molecule contains more than two atoms. An example of the complexities which arise with polyatomic molecules is molecular geometry: how are the atoms in the molecule arranged with respect to one another? In a diatomic molecule, only a single molecular geometry is possible since the two atoms must lie on a line. However, with a triatomic molecule three atoms , there are two possible geometries: the atoms may lie on a line, producing a linear molecule, or not, producing a bent molecule.
Ion 5g light golden brown
The Lewis structure of PCl3 consists of a central phosphorus atom P and three external chlorine atoms Cl. View Result. For the PCl3 molecule, the total number of electron pairs is Dec 22, PCl3 is a polar molecule due to the presence of a lone pair of electrons at the top of the molecule leading to electron-electron repulsion. Download Now. PCl3 is a polar molecule due to the presence of a lone pair of electrons at the top of the molecule leading to electron-electron repulsion. The PCl3 Lewis structure is shown below: Steps for drawing the PCl3 Lewis structure Step 1 Calculate the number of valence electrons for P and Cl Phosphorus and chlorine are elements of group 15 and 17 of the periodic table, respectively. When we talk about the hybridization of PCl 3 students should not confuse it with PCl5. These hybrid orbitals overlap with the p orbitals of chlorine atoms, creating sigma bonds and maintaining the trigonal pyramidal molecular shape of PCl3. XeF2 Lewis structure: drawing, hybridisation, geometry. All in all, if we look at PCl3, there are 3 electron pairs, 3 bond pairs and one lone pair of electrons.
Phosphorus trichloride PCl3 has the composition of one phosphorus and three chlorine atoms. What is the molecular geometry of phosphorus trichloride?. Drawing and predicting the PCl3 molecular geometry is very easy by following the given method.
The central atom must be highly or minimally electronegative. Start Quiz. XeF2 Lewis structure: drawing, hybridisation, geometry. For the PCl3 molecule, the total number of electron pairs is In step 3, for the PCl3 molecule, we can see that there are three lone pairs of electrons on each of the outer chlorine atoms, forming an octet, so they are stable. Looking at the PCl 3 molecular geometry it is trigonal pyramidal with a bond angle of approx. One of the hybrid orbitals will contain one lone pair of electrons. Download Now. Phosphorus and chlorine are elements of group 15 and 17 of the periodic table, respectively. These hybrid orbitals overlap with the p orbitals of chlorine atoms, creating sigma bonds and maintaining the trigonal pyramidal molecular shape of PCl3. In this configuration, the central phosphorus atom is bonded to three chlorine atoms, and an unshared pair of electrons on phosphorus creates a pyramidal shape. Order: 1bag Purity: 99 Supply Ability: Phosphorus in PCl3 undergoes sp3 hybridization, involving the combination of one s orbital and three p orbitals of phosphorus to form four sp3 hybrid orbitals. Further, each chlorine atom also holds 3 lone pairs.

Has casually come on a forum and has seen this theme. I can help you council.
It is removed (has mixed section)
What charming message