P h2o h3po4 h2
Phosphoric acid orthophosphoric acid, monophosphoric acid or phosphoric V acid is a colorless, odorless phosphorus -containing solidand inorganic compound with the chemical formula H p h2o h3po4 h2 P O 4. It is a major industrial chemical, being a component of many fertilizers.
Perchloric acid. HClO 4. ClO 4 -. Perchlorate ion. Hydroiodic acid.
P h2o h3po4 h2
Phosphorous acid or phosphonic acid is the compound described by the formula H 3 PO 3. This acid is diprotic readily ionizes two protons , not triprotic as might be suggested by this formula. Phosphorous acid is an intermediate in the preparation of other phosphorus compounds. Organic derivatives of phosphorous acid, compounds with the formula RPO 3 H 2 , are called phosphonic acids. In contrast, arsenous acid 's major tautomer is the trihydroxy form. On an industrial scale, the acid is prepared by hydrolysis of phosphorus trichloride with water or steam: [5]. HPO OH 2 could be produced by the hydrolysis of phosphorus trioxide :. Phosphorous acid has a p K a in the range 1. The hydrogen atom bonded directly to the phosphorus atom is not readily ionizable. Chemistry examinations often test students' appreciation of the fact that not all three hydrogen atoms are acidic under aqueous conditions, in contrast with H 3 PO 4. This reaction is used for laboratory-scale preparations of PH 3.
On an industrial scale, the acid is prepared by hydrolysis of phosphorus trichloride with water or steam: [5]. Silica is also added, resulting in the production of calcium silicate slag.
Our percent yield calculator will help you to understand how to calculate the percent yield , as well as teach you the percent yield formula and the percent yield definition. Finding the yield is an integral part of any kind of synthetic lab work as the percent yield equation turns your experimental yields into a representation of how successfully you carried out your reaction. Hopefully, after reading this page, you will have an answer to the questions "what is percent yield? What is the percent yield? The percent yield definition is that it is a measure of the effectiveness of a synthetic procedure. Wordy, right?
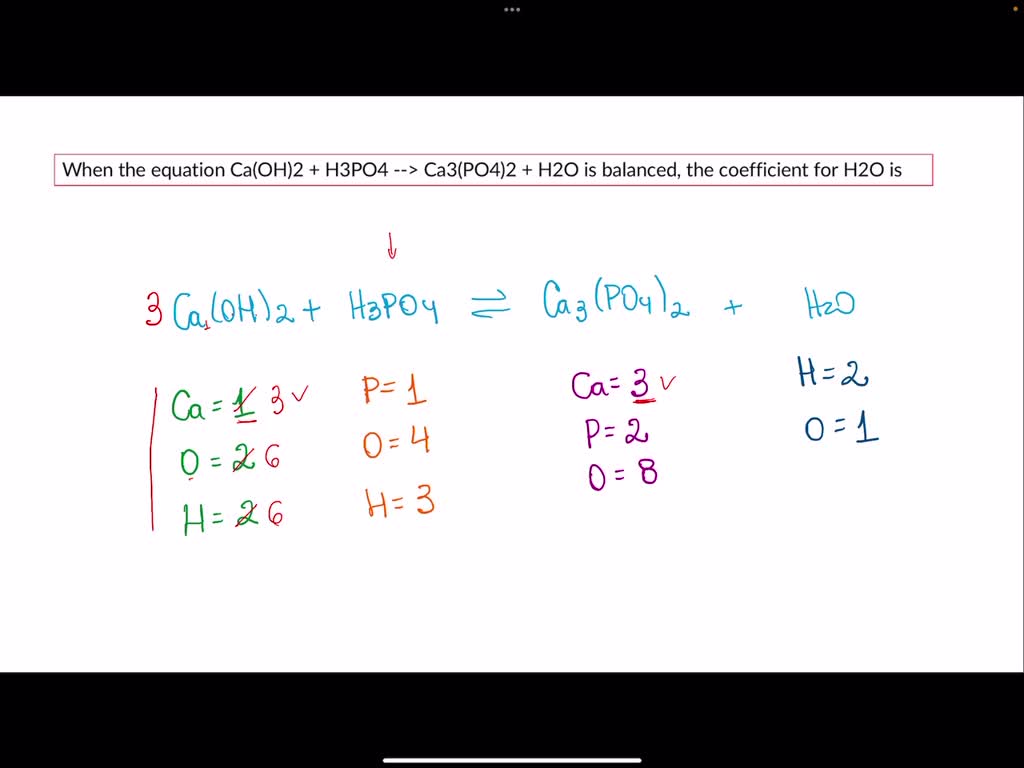
Direct link to this balanced equation:. A chemical equation represents a chemical reaction. It shows the reactants substances that start a reaction and products substances formed by the reaction. However, this equation isn't balanced because the number of atoms for each element is not the same on both sides of the equation. A balanced equation obeys the Law of Conservation of Mass, which states that matter is neither created nor destroyed in a chemical reaction. This is the most straightforward method. It involves looking at the equation and adjusting the coefficients to get the same number of each type of atom on both sides of the equation. Process: Start with the most complex molecule or the one with the most elements, and adjust the coefficients of the reactants and products until the equation is balanced.
P h2o h3po4 h2
.
Utahjaz video
Fluoride ion. Interactive image. PubChem CID. Oh no! If you do not know what products are, enter reagents only and click 'Balance'. PEL Permissible. There are 4 atoms on the left and 1 on the right, let's put 4 in front of H 3 PO 4. Annals of Internal Medicine. In the wet process, a phosphate-containing mineral such as calcium hydroxyapatite and fluorapatite are treated with sulfuric acid. Hydrogen sulfite ion.
.
The phosphoric acid from both processes may be further purified by removing compounds of arsenic and other potentially toxic impurities. Best for: Equations that are more complex and not easily balanced by inspection. Still confused about how to find the percent yield? It reduces solutions of noble metal cations to the metals. Retrieved 11 February CAS Number. Precautionary statements. It is used in the production of basic lead phosphonate PVC stabilizer, aminomethylene phosphonic acid and hydroxyethane diphosphonic acid. Let's try another example to bolster that confidence. Hydrogen sulfite ion. Phosphorous acid slowly oxidizes in air to phosphoric acid. Balancing with inspection or trial and error method This is the most straightforward method. How to calculate percent yield As you may have guessed from the percent yield equation above, if you want to know how to calculate the percent yield, you need two things, your experimental yield, and the theoretical yield. Sulfurous acid.

0 thoughts on “P h2o h3po4 h2”