Number of valence electrons in phosphorus
Valence electrons in a Phosphorus atom Therefore, the valence electron in a Phosphorus atom is 5. Byju's Answer.
Contining on from CHM there are several topics that you must have a firm grasp on in order to be able to understand the concepts being presented in CHM An atom is made up of protons, neutrons and electrons. Protons and Neutrons are located in the nucleus of the atom and electrons are located in shells surrounding the nucleus. An elements atomic number is equal to the number of protons located in its nucleus. If you change the number of protons, you change the element you are talking about. The atomic mass of an element is equal to the mass of its protons plus its neutrons.
Number of valence electrons in phosphorus
Although we have discussed the general arrangement of subatomic particles in atoms, we have said little about how electrons occupy the space about the nucleus. Do they move around the nucleus at random, or do they exist in some ordered arrangement? The modern theory of electron behavior is called quantum mechanics. It makes the following statements about electrons in atoms:. It is the arrangement of electrons into shells and subshells that most concerns us here, so we will focus on that. We use numbers to indicate which shell an electron is in. The first shell, closest to the nucleus and with the lowest-energy electrons, is shell 1. This first shell has only one subshell, which is labeled s and can hold a maximum of 2 electrons. We combine the shell and subshell labels when referring to the organization of electrons about a nucleus and use a superscript to indicate how many electrons are in a subshell. Thus, because a hydrogen atom has its single electron in the s subshell of the first shell, we use 1 s 1 to describe the electronic structure of hydrogen. This structure is called an electron configuration. Electron configurations are shorthand descriptions of the arrangements of electrons in atoms. Helium atoms have 2 electrons.
The d subshell can hold a maximum of 10 electrons. Phosphorus is in group VA so it has 5 valence electrons and Oxygen is in group VIA so each oxygen has 6 valence electrons. BUT wait a minute, that is an odd number of electrons and we haven't really discussed that issue so something must be missing
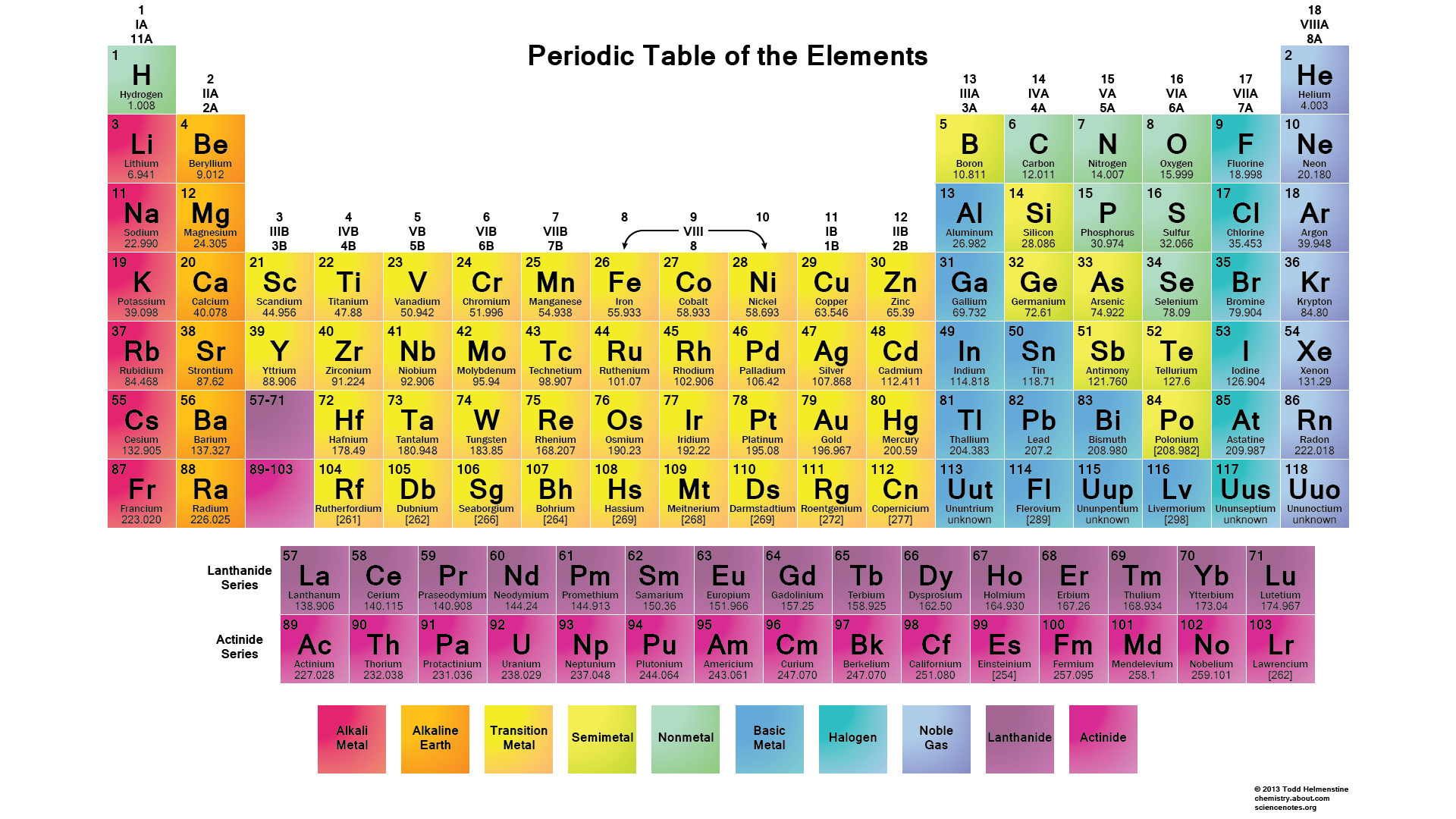
There are two ways to find out. Either you take a look at your periodic table and look at which group number P belongs this can be seen on upper portion or you can draw the electron configuration of P atom based on its atomic number which is According to the periodic table above, phosphorus belongs to Group 5A. Therefore, Its valence electrons should be 5. Thus, valence electrons for P is 5. How many valence electrons does phosphorus have?
Today we shall discuss the valency of another important element, i. As a chemistry student, you need to remember the valency of all the important elements in the periodic table. Phosphorous is a waxy red or white coloured element. Since it is a highly reactive element, it is never found free in nature and is mostly found in the oxidized state in phosphate rocks. Phosphorous shows valencies of both 3 and 5.
Number of valence electrons in phosphorus
The fifteenth element of the periodic table is phosphorus. Phosphorus forms bonds through its valence electrons. The second element in group is phosphorus. The valence electron is the total number of electrons in the last orbit. The total number of electrons in the last shell after the electron configuration of phosphorus is called the valence electrons of phosphorus P. The valence electrons determine the properties of the element and participate in the formation of bonds.
5 letter words with api
Thus, it is convenient to separate electrons into two groups. Other basic trends that you should be aware of are trends in ionization energy and atomic radius. The d subshell can hold a maximum of 10 electrons. Solution The highest-numbered shell is the third shell, which has 2 electrons in the 3 s subshell and 3 electrons in the 3 p subshell. With neon, the 2 p subshell is completely filled. Valence electrons in a Phosphorus atom The most electronegative element is Fluorine and the trend of electronegativity increases from left to right and from bottom to top in the periodic table. How many valence electrons are in an atom of phosphorus? Solution A neutral phosphorus atom has 15 electrons. How many valence electrons does an atom of lithium possess? Negatively charged atoms are called anions and positively charged atoms are called cations. There are two ways to find out. These valence electrons can form a chemical bond only if the outer shell remains unclosed.
If you want a Periodic table with Valence electrons, then visit Periodic table with Valence electrons labeled in it.
We combine the shell and subshell labels when referring to the organization of electrons about a nucleus and use a superscript to indicate how many electrons are in a subshell. Nikka C. Helium atoms have 2 electrons. Thus, valence electrons for P is 5. From the mass in the periodic table and the atomic number, you should be able to determine the number of neutrons in the atom. Explanation: There are two ways to find out. Valence shell electrons or, more simply, the valence electrons are the electrons in the highest-numbered shell, or valence shell, while core electrons are the electrons in lower-numbered shells. You can think of it as a popularity contest and the most electronegative atom is the most popular. Related questions How do valence electrons affect chemical bonding? We can see from the electron configuration of a carbon atom—1 s 2 2 s 2 2 p 2 —that it has 4 valence electrons 2 s 2 2 p 2 and 2 core electrons 1 s 2. How many valence electrons are present in sulphur atom? If you change the number of protons, you change the element you are talking about. Ions form to increase the stability of the atom.

You are mistaken. Write to me in PM, we will communicate.
It is a pity, that now I can not express - there is no free time. I will return - I will necessarily express the opinion.