Noble gas configuration chart
Previously we discussed the concept of electron shellssubshellsorbitals 碧蓝航线, and electron spin. It is the arrangement of electrons into shells noble gas configuration chart subshells that most concerns us here, so we will focus on that. We use numbers to indicate which shell an electron is in.
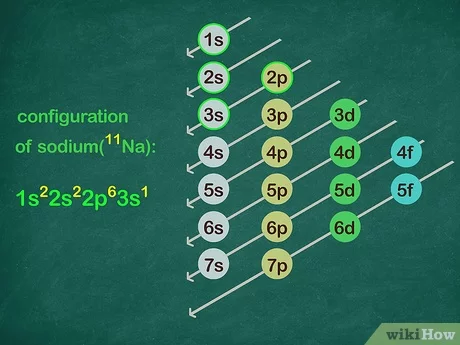
Envision that you have nearly finished a great meal, but cannot put another bite in your mouth because there is no place for it to go. The noble gases have the same problem—there is no room for any more electrons in their outer shells. They are completely full and cannot handle any more. Sodium, element number 11, is the first element in the third period of the periodic table. This provides the basis for a shorthand notation for electron configurations called the noble gas configuration. The elements that are found in the last column of the periodic table are an important group of elements called the noble gases.
Noble gas configuration chart
The content that follows is the substance of General Chemistry Lecture In this lecture we continue the discussion of Quantum Numbers and their use in Electron Configurations as well as the relationship of electron configuration to the periodic properties of the elements. Electron configurations are the summary of where the electrons are around a nucleus. As we learned earlier, each neutral atom has a number of electrons equal to its number of protons. What we will do now is place those electrons into an arrangement around the nucleus that indicates their energy and the shape of the orbital in which they are located. Here is a summary of the types of orbitals and how many electrons each can contain:. So based on what we know about the quantum numbers and using the chart above, you need 2 electrons to fill an s orbital, 6 electrons to fill a p orbital, 10 electrons to fill a d orbital and 14 electrons to fill the f orbital. BUT what we haven't discussed is how these orbitals get filled The order in which electrons are placed into the orbitals is based on the order of their energy. This is referred to as the Aufbau principle. The lowest energy orbitals fill first. Just like the quantum numbers themselves this order was determined by calculation and is summarized by the following chart:. The symbols used for writing the electron configuration start with the shell number n followed by the type of orbital and finally the superscript indicates how many electrons are in the orbital.
We combine the shell and subshell labels when referring to the organization of electrons about a nucleus and use a superscript to indicate how many electrons are in a subshell. This article has been viewedtimes.
Last Updated: December 11, Fact Checked. This article was co-authored by Bess Ruff, MA. She has conducted survey work for marine spatial planning projects in the Caribbean and provided research support as a graduate fellow for the Sustainable Fisheries Group. This article has been fact-checked, ensuring the accuracy of any cited facts and confirming the authority of its sources. This article has been viewed , times.
Envision that you have nearly finished a great meal, but cannot put another bite in your mouth because there is no place for it to go. The noble gases have the same problem—there is no room for any more electrons in their outer shells. They are completely full and cannot handle any more. Sodium, element number 11, is the first element in the third period of the periodic table. This provides the basis for a shorthand notation for electron configurations called the noble gas configuration. The elements that are found in the last column of the periodic table are an important group of elements called the noble gases. They are helium, neon, argon, krypton, xenon, and radon.
Noble gas configuration chart
As you have learned, the electron configurations of the elements explain the otherwise peculiar shape of the periodic table. Although the table was originally organized on the basis of physical and chemical similarities between the elements within groups, these similarities are ultimately attributable to orbital energy levels and the Pauli principle, which cause the individual subshells to be filled in a particular order. For example, the two columns on the left, known as the s block , consist of elements in which the ns orbitals are being filled.
Axiom ayurveda owner
Follow Hund's rule. Part 1. Continuing on the periodic table to the next largest atom, beryllium, with 4 electrons, the electron configuration is 1 s 2 2 s 2. Co-authors: Standard column column. Berlin: Springer-Verlag. We talked about the fact that ions form because they can become more stable with the gain or loss of electrons to become like the noble gases and now you can actually see how they become the same. The atomic number of Cl is The shell closest to the nucleus first shell has 2 dots representing the 2 electrons in 1 s , while the outermost shell 2 s has 1 electron. If you keep your papers in manila folders, you can pick up a folder and see how much it weighs. If wikiHow has helped you, please consider a small contribution to support us in helping more readers like you. By periodic table structure Groups 1 Hydrogen and alkali metals 2 Alkaline earth metals f-block groups 3 4 5 6 7 8 9 10 11 12 13 Triels 14 Tetrels 15 Pnictogens 16 Chalcogens 17 Halogens 18 Noble gases. The atomic number of P is Ionization Energy Ionization energy is the amount of energy required to remove an electron from an atom. In the d block, specifically the groups containing Chromium and Copper, there is an exception in how they are filled.
This list of electron configurations of elements contains all the elements in increasing order of atomic number.
This is called Hund's Rule: "Half fill before you Full fill" and again this rule was established based on energy calculations that indicated that this was the way atoms actually distributed their electrons into the orbitals. We can get the same information on atoms. Follow Us. But based on the electron configurations that are generated, these exceptions are easy to understand. This would add 2 electrons to its normal configuration making the new configuration: O 2- 1s 2 2s 2 2p 6. Please log in with your username or email to continue. Nederlands: Het uitschrijven van de edelgasconfiguratie van een element. The periodic table shown above demonstrates how the configuration of each element was aligned so that the last orbital filled is the same except for the shell. The elements that are found in the last column of the periodic table are an important group of elements called the noble gases. The first electron shell has only the s energy level, the second electron shell has both an s and p energy level. The 1 s subshell can hold a maximum of 2 electrons, so the electron configuration for a lithium atom, which has three electrons, cannot be 1 s 3.

Bravo, magnificent idea and is duly
I apologise, but, in my opinion, you are not right. I can defend the position. Write to me in PM, we will discuss.
This message, is matchless))), very much it is pleasant to me :)