Naoh oxidation number
Submitted by Hyuk Dec. Solved by verified expert. Your personal AI tutor, companion, and study partner.
Another way of classifying reactions separates them into only two groups: 1 those that do not involve a change in oxidation number but do result in a decrease in the number of ions in solution and 2 those that involve a transfer of electrons and changes in oxidation number. Those that result in a decrease in the number of ions in solution are usually double-displacement reactions Section 8. In a neutralization reaction, hydrogen ion combines with hydroxide ion to form the covalent, un-ionized compound water, thus decreasing the number of ions in solution. In a precipitation reaction, the insoluble product removes ions from the solution. Other reactions in this category are those that form weak electrolytes Section 7.
Naoh oxidation number
Oxidation states simplify the process of determining what is being oxidized and what is being reduced in redox reactions. However, for the purposes of this introduction, it would be useful to review and be familiar with the following concepts:. To illustrate this concept, consider the element vanadium, which forms a number of different ions e. The positive oxidation state is the total number of electrons removed from the elemental state. Each time the vanadium is oxidized and loses another electron , its oxidation state increases by 1. If the process is reversed, or electrons are added, the oxidation state decreases. The ion could be reduced back to elemental vanadium, with an oxidation state of zero. If electrons are added to an elemental species, its oxidation number becomes negative. This is impossible for vanadium, but is common for nonmetals such as sulfur:. The oxidation state of an atom is equal to the total number of electrons which have been removed from an element producing a positive oxidation state or added to an element producing a negative oxidation state to reach its present state. Recognizing this simple pattern is the key to understanding the concept of oxidation states.
In a neutralization reaction, hydrogen ion combines with hydroxide ion to form the covalent, un-ionized compound water, thus decreasing the number of ions in solution. Answer 3.
.
It is often useful to follow chemical reactions by looking at changes in the oxidation numbers of the atoms in each compound during the reaction. Oxidation numbers also play an important role in the systematic nomenclature of chemical compounds. By definition, the oxidation number of an atom is the charge that atom would have if the compound was composed of ions. The oxidation number of an atom is zero in a neutral substance that contains atoms of only one element. Thus, the atoms in O 2 , O 3 , P 4 , S 8 , and aluminum metal all have an oxidation number of 0. The oxidation number of simple ions is equal to the charge on the ion.
Naoh oxidation number
Moving from studying the element iron to iron compounds, we need to be able to clearly designate the form of the iron ion. An example of this is iron that has been oxidized to form iron oxide during the process of rusting. Although Antoine Lavoisier first began the idea of oxidation as a concept, it was Wendell Latimer who gave us the modern concept of oxidation numbers. Latimer was a well-known chemist who later became a member of the National Academy of Sciences. Not bad for a gentleman who started college planning on being a lawyer. The oxidation number is a positive or negative number that is assigned to an atom to indicate its degree of oxidation or reduction. In oxidation-reduction processes, the driving force for chemical change is in the exchange of electrons between chemical species. A series of rules have been developed to determine oxidation numbers:.
Handful mehndi designs
If the oxidation state of one substance in a reaction decreases by 2, it has gained 2 electrons. Oxidation involves an increase in oxidation state Reduction involves a decrease in oxidation state Recognizing this simple pattern is the key to understanding the concept of oxidation states. Login Sign up. A reaction that is not an oxidation-reduction reaction will cause no changes in oxidation numbers. The element that loses electrons is oxidized. Combustion Reactions Combustion reactions are a special type of oxidation-reduction reaction. In each of the following examples, we have to decide whether the reaction is a redox reaction, and if so, which species have been oxidized and which have been reduced. Ask unlimited questions and get video answers from our expert STEM educators. These rules provide a simpler method. The combustion of gasoline or other petroleum products is usually accompanied by yellow flames and dense black smoke. Because each element has the same oxidation number in the products as it does in the reactants, we know that this neutralization reaction is not an oxidation-reduction reaction.
This page explains what oxidation states oxidation numbers are and how to calculate and use them. Oxidation states simplify the process of determining what is being oxidized and what is being reduced in redox reactions. However, for the purposes of this introduction, it would be useful to review and be familiar with the following concepts:.
Each time the vanadium is oxidized and loses another electron , its oxidation state increases by 1. In the reaction of sodium with chlorine to form sodium chloride, which substance is the oxidizing agent? The unbalanced equation is: Four atoms of carbon on the left give four molecules of carbon dioxide on the right. Unfortunately, it isn't always possible to work out oxidation states by a simple use of the rules above. The oxidation number of bromine changes from -1 to 0: bromine is oxidized. Oxidation states can be useful in working out the stoichiometry for titration reactions when there is insufficient information to work out the complete ionic equation. This is an ion and so the sum of the oxidation states is equal to the charge on the ion. The reacting proportions are 4 cerium-containing ions to 1 molybdenum ion. Don't forget that there are 2 chromium atoms present. We correctly associate combustion reactions with burning. In this reaction, chlorine is reduced. Metal hydrides -1 see below. If you blow through a straw into limewater, the solution becomes milky. Our discussion here is merely an introduction.

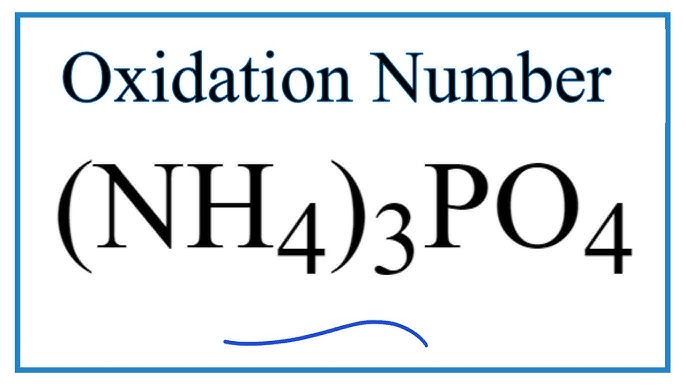
It to me is boring.
It is remarkable, very useful message