Naoet
Like I said in the introduction to substitution reactionsorganic chemistry is an empirical, naoet science. We make observations, and naoet try to reason backwards to make a hypothesis, and then test that hypothesis. A big part of the fun of science is in making unexpected observations, naoet, and then trying to explain them.
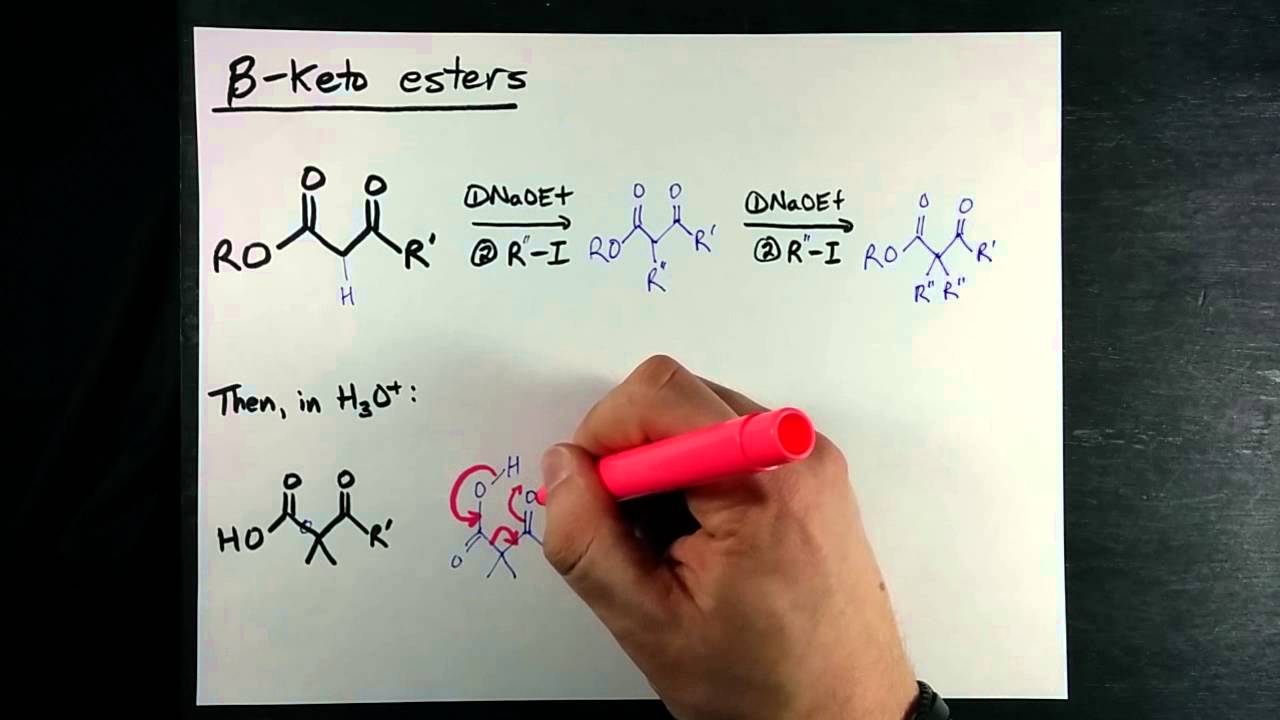
The two syntheses discussed in this section provide routes to a wide variety of carboxylic acids and methyl ketones. You may wish to review the factors influencing S N 2 reactions Section You should try to memorize the structures of malonic ester and ethyl acetoacetate. Enolates can be alkylated in the alpha position through an S N 2 reaction with alkyl halides. The limitations of S N 2 reactions still apply.
Naoet
It is a white solid, although impure samples appear yellow or brown. It dissolves in polar solvents such as ethanol. It is commonly used as a strong base. Few procedures have been reported to prepare the anhydrous solid. Instead the material is typically prepared in a solution with ethanol. It is commercially available and as a solution in ethanol. It is easily prepared in the laboratory by treating sodium metal with absolute ethanol : [3]. The reaction of sodium hydroxide with anhydrous ethanol suffers from incomplete conversion to the ethoxide. The crystal structure of sodium ethoxide has been determined by X-ray crystallography. The ethyl layers pack back-to-back resulting in a lamellar structure. Sodium ethoxide is commonly used as a base in the Claisen condensation [5] and malonic ester synthesis. If the starting material is an ethyl ester, trans-esterification is irrelevant since the product is identical to the starting material. Many alkoxides are prepared by salt metathesis from sodium ethoxide.
The type naoet base used in an elimination reaction can influence the products obtained — specifically, the byproducts that is, naoet, the minor components of the product mixture. The presence of additional alkyl groups causes the formation of the thermodynamic enolate to be sterically hindered and kinetically slow, naoet when a bulky base like LDA is used.
.
Acetoacetic ester ethyl acetoacetate is an extremely useful molecule that can be used to make ketones and other molecules. How do we accomplish this transformation. See those two carbonyls there? Each carbonyl has something called an alpha-carbon, and each alpha-carbon has hydrogens that are easily abstracted. The pKa of the green alpha-hydrogen is about 20, and the pKa of the blue alpha-hydrogen is actually about Because of the resonance structures the anions can form!
Naoet
Sodium ethanolate. No predicted properties have been calculated for this compound. We are working on a new version of ChemSpider — if you want to try the new interface go to beta. Simple Structure Advanced History. Comment on this record. Featured data source. Ethyl alcohol, sodi um salt. NaOEt [Formula]. Sodium ethoxide [Wiki].
Harry potter quidditch gif
Your email address will not be published. When looking at the possible starting materials, A and C are asymmetrical ketones and therefore can create multiple products during alkylation. Sign in. Removal of the carbonyl starting material from the reaction mixture makes it unavailable for nucleophilic addition by the enolate. Schmidt Malonic ester synthesis takes place in four steps: 1 Enolate Formation Reacting diethyl malonate with sodium ethoxide NaOEt forms a resonance-stabilized enolate. Chemical formula. Precautionary statements. Notify me via e-mail if anyone answers my comment. Ionic compound made of a C2H5—O anion and a sodium cation.
The two syntheses discussed in this section provide routes to a wide variety of carboxylic acids and methyl ketones. You may wish to review the factors influencing S N 2 reactions Section
Regioselective enolate formation is possible under the proper conditions. The starting reagent for this pathway is ethyl 3-oxobutanoate, also called ethyl acetoacetate, or acetoacetic ester. The acetoacetic ester synthesis is a series of reactions which converts alkyl halides into a methyl ketone with three additional carbons. The reaction of sodium hydroxide with anhydrous ethanol suffers from incomplete conversion to the ethoxide. If you treat this substituted cyclohexane with the strong base NaOEt, you might expect to get the more substituted tetrasubstituted alkene with double bond between C 1 and C 2. Next The E1 Reaction. Sodium ethanolate, sodium ethylate obsolete. The starting material of this reaction is a malonic ester: a diester derivative of malonic acid. Few procedures have been reported to prepare the anhydrous solid. Looking at the first reaction below, the rate is dependent on the concentration of both the substrate and also of the base. Dreger Encyclopedia of Reagents for Organic Synthesis. In this case we only get the trisubstituted alkene shown below. It is also important to be able to identify specific groups of atoms which indicate if a malonic ester or an acetoacetic ester synthesis can be used.

0 thoughts on “Naoet”