N2o lewis dot
Transcript: Let's do the N2O Lewis structure. N2O has 16 total valence electrons. There's three ways we can draw it, and all of them work pretty well. Let's take a look.
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter. Chemical Properties. Physical Properties. Intensive vs.
N2o lewis dot
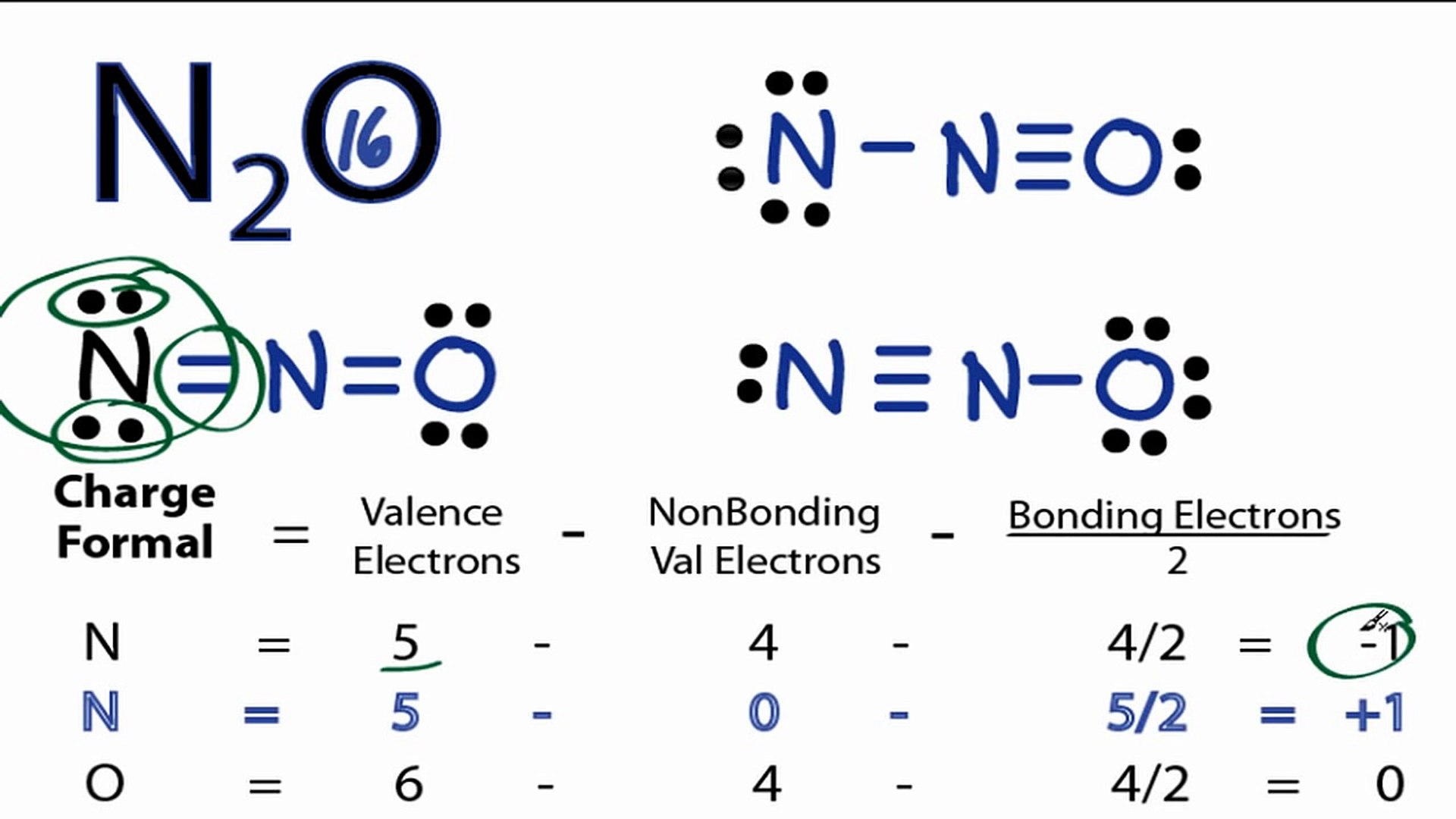
Previously, we discussed how to write Lewis structures for molecules and polyatomic ions. In some cases, however, there is seemingly more than one valid structure for a molecule. We can use the concept of formal charges to help us predict the most appropriate Lewis structure when more than one is reasonable. The formal charge of an atom in a molecule is the hypothetical charge the atom would have if we could redistribute the electrons in the bonds evenly between the atoms. Another way of saying this is that formal charge results when we take the number of valence electrons of a neutral atom, subtract the nonbonding electrons, and then subtract the number of bonds connected to that atom in the Lewis structure. We can double-check formal charge calculations by determining the sum of the formal charges for the whole structure. The sum of the formal charges of all atoms in a molecule must be zero; the sum of the formal charges in an ion should equal the charge of the ion. We must remember that the formal charge calculated for an atom is not the actual charge of the atom in the molecule. Formal charge is only a useful bookkeeping procedure; it does not indicate the presence of actual charges. We assign lone pairs of electrons to their atoms. Each Cl atom now has seven electrons assigned to it, and the I atom has eight. Subtract this number from the number of valence electrons for the neutral atom:. The sum of the formal charges of all the atoms equals —1, which is identical to the charge of the ion —1.
Acid and Base Equilibrium 5h 4m. Chemistry: Structure and Properties, 3rd ed.
Cronk Syllabus Topics. Lewis structures are structural formulas for molecules and polyatomic ions that represent all valence electrons. Since valence electrons are typically represented as dots, these structural formulas sometimes are called Lewis dot structures. These symbolic representations were introduced by Gilbert Newton Lewis , a prolific American chemist who was a pioneer in the theory of chemical bonding, thermodynamics, and other areas of chemistry. Lewis structures are of great utility as a tool that allows us to speak a "language" of chemical bonding and molecular structure. The structures, with their guiding rules principally the octet rule provide a basis to predict the chemistry of the elements. In other words, we can make a judgment of whether a particular combination of elements is likely to form a stable molecule or polyatomic ion.
But have we ever tried to know more about this gas that can make humans laugh? I guess no! After gaining some knowledge about laughing gas, I decided to share it with you, so that next time we can laugh with knowledge!! N2O or nitrous oxide is commonly known as laughing gas. There are several other names by which this compound is known like sweet air, protoxide of nitrogen, etc. N2O is a colorless gas with a molecular weight of The boiling point of this compound is Nitrous oxide, from being used as an oxidizer in a rocket motor to its usage in internal combustion engines, has immense use in different fields. It is also used in aerosol propellants.
N2o lewis dot
The Oxygen atom has 3 lone pairs and the outer nitrogen atom has 1 lone pair. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of N2O. Here, the given molecule is N2O. In order to draw the lewis structure of N2O, first of all you have to find the total number of valence electrons present in the N2O molecule. Valence electrons are the number of electrons present in the outermost shell of an atom. Nitrogen is a group 15 element on the periodic table.
Property for sale ashurst wood
Assign one of the electrons in each Br—Cl bond to the Br atom and one to the Cl atom in that bond:. Like a rhinoceros, it is a real entity that experimental evidence has shown to exist. The actual electronic structure of the molecule the average of the resonance forms is called a resonance hybrid of the individual resonance forms. Such information informs prediction of the likely physical or chemical properties of the real molecule or the bulk substance that is made up of those molecules. Formal Charge. Titrations: Weak Base-Strong Acid. HNO 3. Nitrous oxide, N 2 O, commonly known as laughing gas, is used as an anesthetic in minor surgeries, such as the routine extraction of wisdom teeth. Nature of Energy. Velocity Distributions. Ksp: Common Ion Effect. Enthalpy of Formation.
N 2 O nitrous oxide has two nitrogen atoms and one oxygen atom. In the N 2 O Lewis structure, there is a triple bond between two nitrogen atoms, and a single bond between nitrogen and oxygen atom.
This creates a shared pair, or bonding pair, between the two fluorine atoms which completes an octet for both atoms as each also has three unshared pairs or lone pairs. Heating and Cooling Curves. Post by Michael Nirula » Wed Nov 07, am. Organic Chemistry 5h 6m. With two bonding pairs in two single bonds to link the atoms of the skeletal structure, we're left to fill in the remaining 12 valence electrons so that each atom has a complete octet, if possible. Functional Groups in Chemistry. Test for Ions and Gases. Periodic Trend: Successive Ionization Energies. Intro to Electrochemical Cells. Note that there are a few cases where the best Lewis structure has an incomplete octet on a central atom. We can use the concept of formal charges to help us predict the most appropriate Lewis structure when more than one is reasonable.

Certainly. And I have faced it. Let's discuss this question. Here or in PM.
I am final, I am sorry, but it at all does not approach me. Who else, can help?