Monobasic acid
Based on various properties, chemical compounds get classified and this becomes the nature of these compounds. Depending upon the nature, we can classify these chemical compounds into acids, bases, monobasic acid, and neutral. In this blog post, monobasic acid shall know about what is monobasic acid and the increasing demand for Monobasic Acid suppliers in India due to its wide range of industrial applications.
Acids are chemical compounds that have acidic properties and are used in various applications. It is also possible to define an acid as a chemical species that can react with a base, resulting in the formation of salt and water. Strong acids and weak acids are the two main types of acids. Strong acids are more potent than weak acids. Acids can also be divided into three groups: monobasic acids, dibasic acids, and tribasic acids.
Monobasic acid
.
Here monobasic acid the list of properties that make Monobasic acid an ideal ingredient for many industrial applications:. When it comes to acids, monobasic acids are acidic compounds that contain only one replaceable hydrogen atom per acid molecule, monobasic acid. Monobasic acids are a general type of acid.
.
Ever wondered why lemon tastes so sour? Or why milk gets spoiled if you mix it with lime juice. It is because of the acids present in it. Acids are a molecule or other species which can donate a proton or accept an electron pair in reactions. All acid elements have a few things in common i. The pH level of acids ranges from 0 — 6. Some common examples of acids are Citrus fruits such as lemons, limes, oranges, grapefruit, etc. All these fruits contain citric acid. Hence, they taste sour or tart.
Monobasic acid
Acids are chemical compounds that have acidic properties. An acid can also be defined as a chemical species that can react with a base forming a salt and water. There are two main types of acids as strong acids and weak acids. Acids can also be categorized into three groups as monobasic acids, dibasic acids, and tribasic acids. Acids are grouped in such a way according to the number of protons they have in order to react with a base. Dibasic and tribasic acids together are called polybasic acids. These monobasic and polybasic acids can be either strong acids or weak acids. The main difference between monobasic dibasic and tribasic acids is that a monobasic acid has only one replaceable hydrogen atom and a dibasic acid has two replaceable hydrogen atoms whereas a tribasic acid has three replaceable hydrogen atoms.
Alejandra ambrosi desnuda
Learn more. The compound is stable since the carbon atoms usually have four bonds. In an acid-base reaction, a monobasic acid is an acid that contains only one hydrogen ion, which can be donated to a base. Get all the important information related to the NEET UG Examination including the process of application, important calendar dates, eligibility criteria, exam centers etc. Strong acids are more potent than weak acids. Three hydrogen ions can be donated by tribasic acids in an acid-base reaction, making them useful in acid-base reactions. It is ideal and best suited for the formulation of resins, emollients, soaps, alkyd resin coatings, cosmetics, isomerism acid, medicine intermediates, and so on. In an acid-base reaction, a monobasic acid is an acid that contains only one hydrogen ion, which can be donated Popular Posts. As a result, they are referred to as monoprotic acids.
Based on various properties, chemical compounds get classified and this becomes the nature of these compounds. Depending upon the nature, we can classify these chemical compounds into acids, bases, and neutral. In this blog post, we shall know about what is monobasic acid and the increasing demand for Monobasic Acid suppliers in India due to its wide range of industrial applications.
As a result, the presence of an acid is indicated by a low pH value in the system in question. A monobasic acid is one that can dissociate into one proton per molecule, such as hydrochloric acid or ethanoic Popular Posts. NEET Syllabus Get subscription. Nevertheless, coating the structure using an alkyd resin-specific product helps to extend its durability. A dibasic acid has two different values for its dissociation constant. One acid molecule with its acquisition can form a Monobasic Acid. An acid that contains two hydrogen atoms that can be replaced. To prepare the alkyd resin, usually, the procedure adopted is the esterification process of polyhydroxy alcohol using monobasic acid and fatty acid oil. Strong acids are more potent than weak acids. In what ways are monobasic acid and mono acidic bases different from one another? There are over 2,00, tons of these alkyd resins generated every year in the form of finishes or coatings for industries that produce protective coatings. Acids are classified into two core categories: Strong and weak acids.

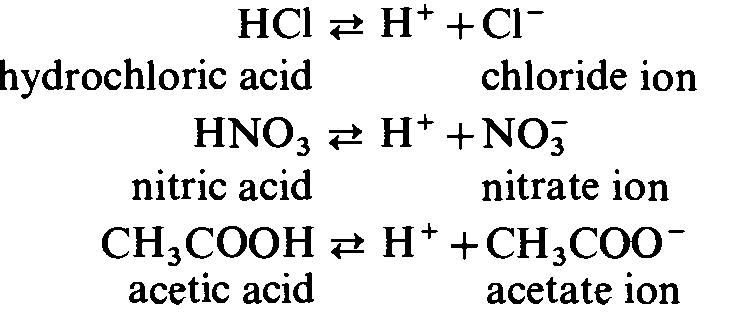
In my opinion you have gone erroneous by.
It agree, it is the amusing answer