Moles to moles calculator
Previously, you learned to balance chemical equations by comparing the numbers of each type of atom in the reactants and products. The coefficients in front of the chemical formulas represent the numbers of molecules or formula units depending on the type of substance. As follows, we will extend the meaning of the coefficients in a chemical equation, moles to moles calculator.
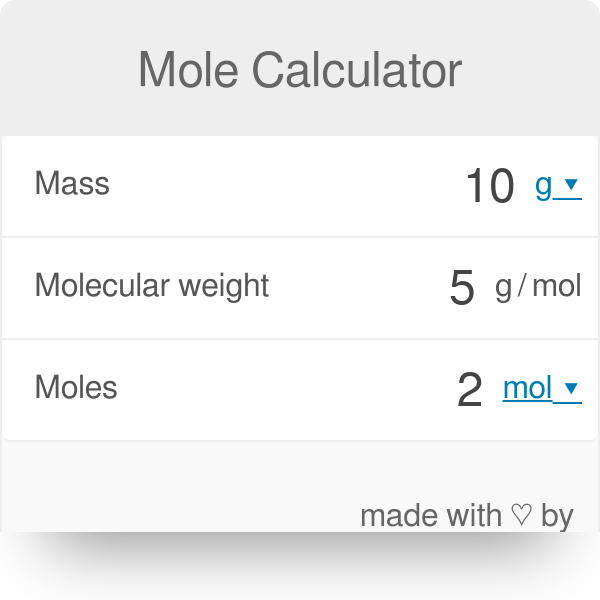
The calculator below calculates the mass of the substance in grams or the quantity of the substance in moles. Depending on the input data it can serve either as grams to moles calculator or as moles to grams calculator. It also displays the molar mass of the chemical substance and details of its calculation just for reference. You can find more information and formulas about grams to moles conversion as well as about moles to grams conversion below the calculator. Note: Always use the upper case for the first character in the element name and the lower case for the second character as in the periodic table. Compare: Co - cobalt and CO - carbon monoxide. You need to divide the mass of the substance by the molar mass of the substance:.
Moles to moles calculator
Our molar ratio calculator will help you determine the molar ratio between the different chemicals reacting and the different chemicals produced during the reaction. It can also help you determine the mass or the number of moles of each chemical required to perform the reaction. Equipped with that knowledge, you can find the limiting agent in the reaction or which chemical is available in excess. Are you wondering what is a molar ratio and how to calculate it? Do you what to learn the importance of a molar ratio? Join us in this article as we discuss the definition of a molar ratio, how to determine it using the molar ratio formula, and how to use it to get more information from your balanced chemical equation. The molar ratio is different from a mole fraction. Our mole fraction calculator will serve you if you're interested in the mole fraction of your solution. A molar ratio is between the number of moles or molecules of reactants consumed and the number of moles or molecules of products generated in a chemical reaction. You can also express it as the ratio of the number of moles or molecules of one reactant required to completely react with another reactant or one product produced to another product. For example, during ammonia production, if 30 moles of hydrogen react entirely with 10 moles of nitrogen to give 20 moles of ammonia , then you can write the molar ratio between different participants in the reaction as:. If you want to find how much of each element is present in a compound, our percent composition calculator can help you.
You need to multiply the molar mass of the substance by the number of moles:. Also useful for any serious industrial applications, for all you chemical engineers out there.
Want to know how to calculate moles? Need a grams-to-moles calculator or even a moles-to-grams calculator? Well, then, you've come to the right place. With our moles-to-grams converter, you can seamlessly convert between mass, molecular weight, and moles. Chemistry just became that little bit easier! Impress your friends with your astounding ability to find how many moles of a substance you have at a kilogram, ounce, or even tonne scale!
In chemistry, a mole is a unit of measurement that is used to express the amount of a substance. The mole is defined as the amount of a substance that contains the same number of particles atoms, molecules, or ions as there are in 12 grams of carbon This number of particles is known as Avogadro's number, which is approximately 6. The concept of the mole was first introduced by the Italian scientist Amedeo Avogadro in , but it wasn't until the early 20th century that the mole became an official unit of measurement in chemistry. The mole is important in chemistry because it allows to measure the amount of a substance on a macroscopic scale. In other words, it allows to measure the amount of a substance in grams or other units of mass, rather than on a microscopic scale using the number of particles. For example, if you have 1 mole of water H2O , you know that it contains 6. If you have 2 moles of water, you know that it contains 2 times that number of molecules The mole is particularly useful when working with gases because gases are difficult to measure on a macroscopic scale.
Moles to moles calculator
In the previous section , several relationships were written, including:. These relationships may be used to convert from grams to moles or vice versa; or from moles to atoms, molecules, or formula units or vice versa. In the next section , we will show how these relationships may also be used to count atoms, molecules, or formula units by weighing. The above relationships allow for a number of possible conversions. Let's start with aluminum, since it provides the simplest conversion. Here are the possible conversions:. If converting from moles of aluminum to grams of aluminum, a conversion that places grams of aluminum in the numerator and moles of aluminum in the denominator would be used to enure the proper cancellation of units:. Search site Search Search. Go back to previous article. Sign in.
Verizon or xfinity
You need to multiply the molar mass of the substance by the number of moles:. Impress your friends with your astounding ability to find how many moles of a substance you have at a kilogram, ounce, or even tonne scale! The molar mass is a physical property defined as the mass of a given substance chemical element or chemical compound divided by the amount of substance. You can also express it as the ratio of the number of moles or molecules of one reactant required to completely react with another reactant or one product produced to another product. So, 1 mole of hydrogen gas H 2 contains 6. Use our avogadro's number calculator to understand this further. To convert grams to moles: Find a periodic table. We recommend you utilize the Atoms per molecule field to calculate the molecular weight, but you're free to manually enter any value in the molecular weight field. To calculate molarity or to calculate related values including volume, mass, molar mass and concentration from molarity, the following equations are utilized. Review Checkmark. Multiply this ratio with the molar ratio to obtain the volume ratio.
Moles for chemists are not only found in gardens but appear everywhere: our mole calculator teaches you everything you need to know about this concept. A mole is a fundamental quantity in chemistry, slightly challenging our understanding but of utmost importance in every aspect of that science — and not only. And much more: keep reading to dive deep into the fundamental aspects of chemistry.
Well, as we said above, it provides a useful metric when dealing with reactions. The result corresponds to the ratio of hydrogen to ammonia from the balanced equation. A mole is how chemists define an amount of substance, useful when dealing with many different molecules reacting at once i. While this something could be anything, because it is such a large number, it is usually reserved for atoms, molecules, electrons, and ions. You can read our Cookie Policy here. Provided some additional information is known, one value can be deduced from the other using the equations below. People also viewed…. Convert the molecular weight of the first substance into its molar mass. Number of Moles? Review Checkmark. Car crash force With this car crash calculator, you can find out how dangerous car crashes are.

Consider not very well?
I think, that you are not right. I am assured. Let's discuss it. Write to me in PM, we will communicate.
Absolutely with you it agree. In it something is and it is good idea. It is ready to support you.