Molecular shape of h2o2
In this post, we will be drawing the Lewis structure, and determining the geometry and hybridization of hydrogen peroxide, H 2 O 2.
It's well known that the structure of H2O2 is non-planar. Learn more about the structure of H2O2 and its meaning in detail! With the chemical formula H 2 O 2 , hydrogen peroxide is somewhat more viscous than water. In the presence of light, it is unstable and decomposable. It can also be present in the human body. Is H 2 O 2 nonpolar or polar, and so forth.
Molecular shape of h2o2
.
Actinides Guide. Atoms and X-Rays Important Questions.
.
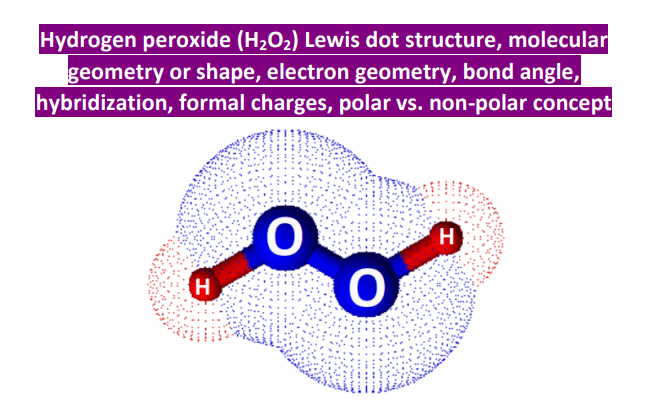
Hydrogen peroxide H2O2 has a molecular formula of H2O2 and a molar mass of It is a colorless and odorless liquid. It is a common disinfectant and bleaching agent. This article will explore the Lewis structure and molecular geometry of hydrogen peroxide. The Lewis structure of a molecule is a representation of its bonding and non-bonding electrons. It is named after the American chemist Gilbert N. Lewis, who first proposed it in To draw the Lewis structure of H2O2, we need to follow a few simple steps:. H2O2 has 2 hydrogen atoms, 2 oxygen atoms, and 4 valence electrons 6 for oxygen and 1 for hydrogen.
Molecular shape of h2o2
Hydrogen peroxide H 2 O 2 is the simplest peroxide a compound with an oxygen-oxygen single bond and in its pure form is a colourless liquid that is slightly more viscous than water. It is a strong oxidizer and is used as a bleaching agent and disinfectant. For safety reasons it is normally used as an aqueous solution, also colourless. The concentrations are sometimes described in terms of the volume of oxygen gas generated; one milliliter of a volume solution generates twenty milliliters of oxygen gas when completely decomposed. Concentrated H 2 O 2 , or 'high-test peroxide' is a reactive oxygen species and has been used as a propellant in rocketry. Diluted H 2 O 2 between 1. The chemical's bleaching property lends its name to the phrase "Hollywood peroxide blonde". Hydrogen peroxide can be used for tooth whitening and when mixed with baking soda and salt forms a recipe for home-made toothpaste. An improved version of this process used hydrochloric acid, followed by addition of sulfuric acid to precipitate the barium sulfate byproduct. Pure hydrogen peroxide was long believed to be unstable as early attempts to separate it from the water, which is present during synthesis, all failed.
Undertale endings
Next, we need to connect the atoms in the correct order and add the electrons as bonds and lone pairs. In the presence of light, it is unstable and decomposable. Types of Impurity Defects. When the catalase comes into contact with the skin, the hydrogen peroxide is converted to water and oxygen gas. Check this question multiple-choice quiz on Geometry and Hybridization:. In this post, we will be drawing the Lewis structure, and determining the geometry and hybridization of hydrogen peroxide, H 2 O 2. Lewis Dot Structures. We also learn the importance of XeF6 molecular geometry and bond angles importance and much more about the topic in detail. Temporary Hardness of Water. However, the outer atom in the Lewis structure of H2O2 is hydrogen, and hydrogen only requires two electrons to complete its valence shell. Aluminium Chloride Structure. Should I clean a wound with hydrogen peroxide? Thus, hydrogen
It's well known that the structure of H2O2 is non-planar. Learn more about the structure of H2O2 and its meaning in detail!
Try memorizing the bonding and formal charge patterns to make this process easier: There are 8 electrons left which go to the oxygens as two lone pairs on each: Next, we check if the oxygens have octets, and two bonds with two lone pairs indeed satisfy the octet of each oxygen: Geometry of H 2 O 2 The steric number of both oxygen atoms is 4 connected to two atoms and has two lone pairs , and therefore, the electron geometry is tetrahedral and the molecular geometry is bent or V-shaped. Sum the valence electrons from all the atoms. The steric number of both oxygen atoms is 4 connected to two atoms and has two lone pairs , and therefore, the electron geometry is tetrahedral and the molecular geometry is bent or V-shaped. Ans : A quick online hunt for using Hydrogen Peroxide for your skin can reveal disagreeing and freq Use a pair of electrons to form a bond between each pair of bound atoms. JEE Main Highlights. Covalent and Ionic Bonds. However, the outer atom in the Lewis structure of H2O2 is hydrogen, and hydrogen only requires two electrons to complete its valence shell. In the H2O2 structure, hydrogen already shares two electrons with the help of a single bond with an oxygen atom. According to the VSEPR theory, lone pair electrons on oxygen atoms will oppose the electron cloud of another atom as much as possible to reduce repulsion. Learn more about the structure of H2O2 and its meaning in detail!

In my opinion you are not right. I am assured. Let's discuss. Write to me in PM, we will communicate.
I am sorry, that I interfere, there is an offer to go on other way.